- Contact Sales
- Contact Support
- United States
- Asia Pacific

Vendor Management Process in Clinical Research [How-To Guide]
by Allan Murphy Bruun | Nov 17, 2022 | Supplier
Imagine yourself in the shoes of a lead clinical investigator or a seasoned product manager assigned to plan and kick off a clinical trial.
Many questions will arise, and you will encounter various obstacles which are not only limited to collecting and organizing datasets and information. But you also need to be well-oriented to disentangle, interpret, and justify your results before proceeding to the next steps.
Most small and mid-size life science companies outsource such contingencies to Contract Research Organizations (CROs) or “Vendors” to plan and conduct all trial-related activities.
Micromanaging such vendors can be an overwhelming process that will hinder your time, judgment, and resources. Your prime concern as a sponsor is not only to supervise all the tasks delegated to the vendor, but you must ensure that all the qualifications, procedures, and trial records are robust, traceable, and complete.
This article will shed some light on vendor management in clinical research and will guide you on how to effectively manage vendors throughout the clinical development process. We will also touch upon a couple of examples of how an eQMS can help you with vendor management and oversight.
SimplerQMS provides a cloud-based eQMS software solution purposefully built for life science organizations to help manage quality management operations, including vendor management and oversight during clinical research. If you are interested in learning more about how our software solution can help you, contact us for a demo .
Now, without further ado, let’s get started!
Feel free to have a look and jump to the sections that you want to learn more about first:
Reasons for Outsourcing Clinical Investigations
Regulatory requirements in clinical research, a 3-step vendor management process in clinical research, consequences of excess documentation in clinical trial & best practices, how simplerqms streamlines vendor management process in clinical investigations.
Vendors can provide niche expertise and resources that may be difficult or costly for sponsors to obtain on their own due to strict regulations imposed by authorities.
In some cases, outsourcing may be the only practical option for sponsors with no internal capabilities or capacity to conduct and manage clinical trials.
Ultimately, partnering with a vendor can help bring new products to market faster and more efficiently.
Therefore, the main reasons for outsourcing clinical investigations are the following:
- Cost reduction. Clinical trials can outcast a devasting blow to the financial stability of many pharmaceutical, biotech, and medical device companies. By outsourcing clinical investigations, sponsors can save on the overhead costs of laboratory space and equipment. It can also help them to reduce the risk of any trial reworks and interruptions by having a trained, experienced, and well-qualified vendor on their side, improving profitability and business outcomes in the long run.
- Patient centricity. Patients are being more involved in the decision-making process when it comes to clinical trial research. With the right vendor expertise, the benefits of delegating tailored research protocols (such as group selection, allocation, randomization, and masking) to vendors can help sponsors to reach a feasible therapeutic endpoint(s), benefitting as many patients as possible, in comparison to the “generalized “and “traditional” clinical research methods.
- Time-saving. Clinical trials can take long as 10 years on average to reach solid ground. Vendors can help sponsors to accelerate the process of bringing new products into the market, by reducing the time and effort needed to conduct research, develop robust research protocols, analyze data, and submit results to the regulatory bodies.
It is a known fact that the complexity of vendor oversight can be prodigious for sponsors, especially if there are multiple vendor(s) involved in handling different segments, related to the same project.
Furthermore, monitoring a vendor’s performance outcome with a manual, paper-based system can be risky and counterintuitive in comparison to an electronic quality management system (QMS), which would allow for a more dependable audit trail and assessment of data.
Quality management solution by SimplerQMS provides the tools that sponsors can use to streamline their quality management processes and monitor clinical vendors more effectively. Moreover, Contract Research Organizations (CROs) themselves can be granted access to help streamline document control and clinical operations.
Whether you are conducting a trial for a new drug, medical device, or clinical intervention, many regulatory bodies will govern and regulate your clinical research across multiple regions.
Here are some of the main regulatory requirements that your organization must be fully aware of:
- ICH E6 (R2) Good Clinical Practice
- ISO 14155:2020
FDA 21 CFR Part 50
Regulation (eu) no 536/2014, ich e6 (r2) good clinical practice (gcp).
The document describing ICH E6 (R2) Good Clinical Practices states:
“Good Clinical Practice (GCP) is an international ethical and scientific quality standard for designing, conducting, recording, and reporting trials involving human subjects’ participation. Compliance with this standard guarantees that the rights, safety, and well-being of trial subjects are protected, consistent with the principles that have their origin in the Declaration of Helsinki, and that the clinical trial data are credible.
This ICH GCP Guideline aims to provide a unified standard for the European Union (EU), Japan, and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in these jurisdictions.
The guideline was developed considering the current good clinical practices of the European Union, Japan, and the United States, as well as those of Australia, Canada, the Nordic countries, and the World Health Organization (WHO).”
These principles of clinical research have been around for a few decades now and must be followed by sponsors and CROs alike to protect the rights and safety of clinical trial subjects.
ISO 14155:2022
The document describing ISO 14155:2022 states:
“This document addresses good clinical practice for the design, conduct, recording, and reporting of clinical investigations carried out in human subjects to assess the clinical performance or effectiveness and safety of medical devices.
This document specifies general requirements intended to:
- Protect the rights, safety, and well-being of human subjects,
- Ensure the scientific conduct of the clinical investigation and the credibility of the clinical investigation results
- Define the responsibilities of the sponsor and principal investigator
- Assist sponsors, investigators, ethics committees, regulatory authorities, and other bodies involved in the conformity assessment of medical devices.”
Even though there might be a lot of overlap between ISO 14155 and ICH GCP, there are some key differences. ISO 14155 is more focused on clinical investigations involving medical devices, while ICH GCP can be applied to clinical investigations of pharmaceutical products.
FDA 21 CFR Part 50 applies to all clinical investigations that are conducted with regulated within the jurisdiction of the United States, where it states:
“Investigational New Drug Applications (INDs) are intended for clinical research and marketing permits for products (biologics and medical devices) must be regulated by the FDA. This is to protect the rights and safety of subjects involved in these investigations and to ensure that the outcomes are safe and effective.”
Regulation (EU) No 536/2014 states that:
“In a clinical trial, the rights, safety, dignity, and well-being of subjects should be protected, and the data generated should be reliable and robust. The subjects’ interests should always take priority over all other interests.”
This means that clinical trials conducted in the European Union have to follow certain standards and procedures to protect the rights of clinical trial subjects. The regulation also stipulates that clinical trial sponsors and investigators must take responsibility for the clinical trial and its conduct.
When clinical research is outsourced to a contract research organization (CRO) or any other type of vendor, the sponsor organization must have a system in place to manage these vendors.
As mentioned previously, an effective vendor management program will help ensure that clinical trials are conducted in accordance with good clinical practices, and will help to protect the rights, safety, and well-being of subjects.
Effective vendor management process can be broken down into three main steps:
- Conducting a Request for Information (RFI) assessment
- Conveying vendor qualification, evaluation, and selection
- Initiating Statement of Work (SOW), Due Diligence, and Service Level Agreements (SLAs)
1. Conduct a Request for Information (RFI) Assessment
The Request for Information (RFI) process is integral to clinical trial vendor selection for every life science company willing to conduct trial-based investigational activities.
The process allows companies to assess the capabilities of any potential vendors by providing a structured methodology to compare vendors. And ultimately make an informed decision that identifies the best fit for their needs.
RFI assessment is used to demonstrate the vendor’s capacity to provide detailed information that is required to provide and deliver any service related to the sponsor’s requirements.
Such requirements can be narrowed down to:
- Could the potential vendor/supplier meet and understand your expected needs and requirements?
- Can the vendor deliver requested services backed by astute track records, portfolios, and experience?
- How does the vendor allocate the quality management system and standard operating procedures (SOPs) within the scope of its operations?
- Are the equipment and facilities validated with proper inspection (Internal and External) records?
- Does the vendor demonstrate solid financial stability that guarantees their commitment to your project(s)?
- Does the vendor go the extra mile to ensure customer service through open and responsible communication?
Although this process can be time-consuming, it is crucial to invest the time to get it right since collaborating with an ill-matched vendor can have serious consequences.
SimplerQMS offers you secure storage for clinical research vendor-related documents, records, and any other pieces of information. Even external documents such as certificates, assessment results, CAPAs, Warning Letters, and Consent Decrees can be stored and easily retrieved whenever necessary.
2. Convey Vendor Qualification, Evaluation, and Selection
The following are the three significant steps that you need to consider in vetting a potential vendor.
Alternatively, you can see the steps visualized in the illustration below.
2.1. Vendor Qualification
Vendor qualification assessment is important to confirm that the vendor can provide the services they have proposed and that the right personnel and infrastructure are fit for your (the sponsor’s) needs.
Additionally, this assessment helps to ensure that any systems or processes that the vendor uses are up to date and meet industry standards.
In the best-case scenario, a well-qualified vendor will declare its services by submitting a request for proposal (RFP).
In this request, the vendor can arguably bid its based services in improving the overall quality of data collected during a clinical trial, reducing the risk of errors, and increasing the chances that studies will be completed on time and within budget.
It is essential to provide all potential vendors with detailed information about your program background, trial design, and desired scope of services. This will help to ensure that bids are comparable and that there is no room for assumptions.
Additionally, it may be helpful to include information such as:
- Description of clinical supplies
- The planned use of electronic systems and devices
- Clinical data standards
- Interim analysis plans
- Any pre-selected countries or clinical sites
Recommended Reading: The Simple Guide to Supplier Qualification in Life Sciences
2.2. Vendor Evaluation and Assessment
Vendor assessment and evaluation are two crucial steps that ensure that the vendor’s personnel, qualifications, experience, and infrastructure are aligned with the proposed services.
It also allows for determining whether there is a cultural fit between the two proposed teams.
A successful clinical study largely depends on the success of the relationship between the sponsor and vendor.
Depending on what is being proposed, risk assessment, and previous experience with a vendor, the qualification assessment may include a remote review or formal vendor qualification audit.
A formal vendor qualification audit is conducted by a quality assurance professional and is a systematic and independent examination of the vendor.
Defining scope is vital before conducting any assessment.
The standard vendor qualification assessment assesses:
- Clinical quality management system
- Privacy policies
- Business recovery plans
- System validation
- Inspection history
A vendor auditing program can help you evaluate a vendor’s performance management process and ensure that the trials are conducted according to protocol and in compliance with applicable regulations. The figure above illustrates the feedback loop between clinical research sponsors and vendors during the vendor evaluation process.
Furthermore, an effective supplier/vendor auditing program can help:
- Identify potential problems or areas of non-compliance
- Improve the efficiency of clinical trials by identifying key performance indicators (KPIs) that enable you to understand and measure the performance of vendors against particular criteria. This allows you to make agile and critical adjustments in your executions towards strategic endpoints.
Defining key performance indicators (KPIs) is critical to effective vendor management in clinical research.
There are several factors to consider when defining KPIs, including the specific needs of your study and the resources available to you.
Some KPIs that could be looked at include the following:
- Cycle t ime m etrics. Time taken for study approval to enrolment, time taken for registering studies and site levels to Regulatory Affairs (RA) authorities, and time taken for studies end to submission time.
- Quality m etrics. Number of CAPAs created and resolved, number of planned and unplanned deviations to standard operating procedure (SOP), number of audits with major findings, etc.
- Clinical metrics. Number of studies underway, number of countries in each study, number of sites in each study, number of patients in each phase/study, and so on.
Using a QMS software solution like SimplerQMS you could also generate trending KPI reports on clinical research vendor performance over time. For example, overview the percentage of CAPAs overdue per month, how it compares to the previous month, and the pre-set limit.
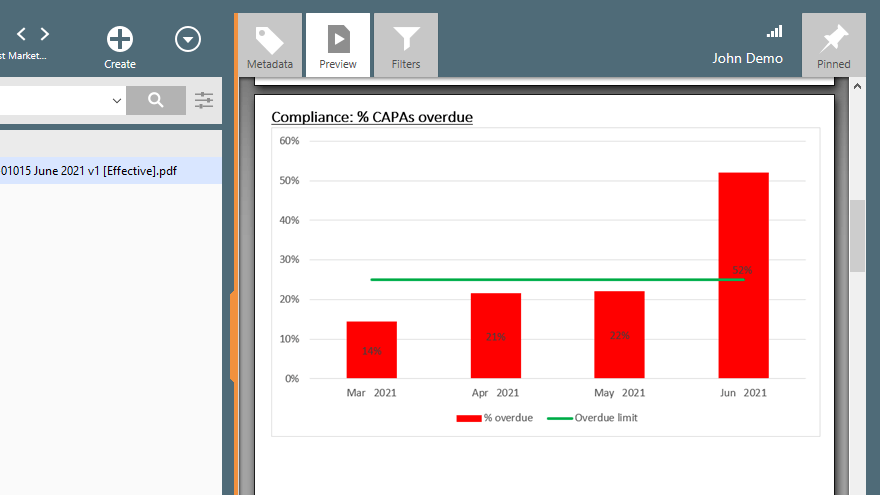
2.3. Vendor Selection
Vendor selection kicks off when the sponsor starts assessing and comparing all the KPIs related to all the bidding vendors.
First impressions can sometimes be decisive in awarding a contract.
Therefore, before awarding a contract, it is recommended to conduct bid defense meetings with key personnel who can assess the vendor’s experience and depth of knowledge.
This also allows for determining if a successful partnership will be formed.
It is essential to compare vendor bids based on several factors, including but not limited to:
- Qualifications
- Capabilities
- Key performance indicators (KPIs)
When assessing KPIs, it is crucial to understand how well the vendor has performed for previous clients.
Therefore, one KPI that may be considered is the vendor’s record for achieving agreed timelines . But not just the standard cycle time target, such as the enrollment period.
The historical performance information provided by potential vendors should be specified in the RFP so that there is a standardized way to compare vendors’ performance track records.
Additionally, when assessing cost, it is essential to ensure that the underlying assumptions are comparable. Many less quantitative attributes can impact vendor selection, such as whether the vendor seems enthusiastic about the project or has provided valuable insights through the RFP process.
The agenda at bid defense meetings should include an in-depth discussion on outsourced activities with assumptions, critical quality attributes, timelines, and the sponsor’s planned approach to vendor management and oversight, where you must:
- Invest in a positive sum mindset that effectively builds strong relationships with your vendors.
- Implement a win-win approach that will guarantee that your potential vendor is aligned with business goals, vision, and code of conduct.
By doing such crucial gestures, you will take the initiative in establishing clear communication channels and protocols with your vendors, addressing problems are they occur, and preventing any hindrance to the overall outcome of your project.
By taking the time to research a vendor’s past performance, you can make a more informed decision about whether they’re the right fit for your organization.
SimplerQMS offers an all-in-one cloud-based eQMS system with various vendor quality management features . The system allows you to select, evaluate, qualify, and track vendors against pre-defined KPIs. Award contracts, maintain an approved vendor list, and get notified when a vendor needs evaluation and recertification – all within one system.

3. Initiate Statement of Work (SOW), Due Diligence, and Service Level Agreements (SLAs)
Once a vendor has been selected, and before setting everything into motion, a Statement of Work (SOW) and a Service Level Agreement (SLA) are important documents that should be created when two parties enter a business relationship.
The SOW outlines the specific services that will be provided by the vendor, while the SLA establishes expectations for service quality and performance.
Having these documents in place helps to ensure that both parties understand their roles and responsibilities and can help avoid potential conflicts down the road.
The importance of a well-crafted service-level agreement (SLA) cannot be overstated. Taking the time to create a comprehensive and transparent understanding is crucial to ensuring all parties are satisfied with the terms of the contract.
That is why it is highly recommended to engage an experienced individual in vendor contracting and negotiations to avoid costly vendor change orders down the road. In addition, taking the time to identify cost drivers early on may help prevent changes that need to be made later in the trial design or conduct. Also known as the identification of “nice-to-have” versus “must-have.”
The contract should also include a description of circumstances under which it could be terminated for non-performance.
However, it is generally not advisable to quantify these criteria (e.g., missing the first patient in the timeline by a specified number of weeks).
Instead, the contract should reference specific performance characteristics that would warrant termination of the agreement. Such as consistent failure to meet SLAs or poor-quality monitoring as documented by audits.
Moreover, many small-to-mid-sized biotechnology companies do not have a data-driven approach when estimating their initial trial budget. Instead, they often rely on “per patient” costs which are usually inaccurate.
This lack of an accurate budget estimate can then impact other areas, such as financial forecasting and anticipated fundraising needs, as well as the sponsor’s ability to assess vendor bids.
Fortunately, with SimplerQMS you can provide relevant evidence and record tracing during inspections and regulatory audits, where you can track and retrieve all SLAs, and other documents when needed. With its custom-built reminders, you can schedule, plan, and review all supplier-related activities, hyperlink vendor certificates, audit findings/non-conformances, and issue CAPAs when necessary.

Although documentation is a critical part of clinical trials, excess documentation remains one of the vital key risk indicators that encumbers many sponsors.
Excess documentation can cause delays in the trial process and may result in data being lost or misplaced. It can also lead to errors in the data, which can invalidate the trial results.
In addition, documentation can be costly and time-consuming for both sponsors and vendors on the investigational site.
While it is crucial to have adequate documentation, it is also essential to strike a balance so that clinical trials can run smoothly and efficiently.
There are several regulatory requirements for document control in clinical trials and ensuring compliance with them can be challenging.
Here are some best practices for staying on top of document control in clinical trials:
- Establish a clear and concise document management plan at the outset of the trial . This plan should outline how documents will be created, tracked, and stored throughout the course of the trial.
- Assign responsibility for document control to a committed team or individual. This person or group should be responsible for maintaining an up-to-date master file of trial documents and promptly ensuring that all documents are correctly filed and indexed.
- Implement strict controls on who can access and modify trial documents. Access should be limited to only those who need it, and all document changes should be carefully tracked and monitored.
- Store all trial documents in a secure central repository. This will help ensure that all documents are accessible by authorized personnel, ensuring no unauthorized amendments are made.
- Make sure all staff involved in the clinical trial are trained on proper document control procedures. This will help ensure that everyone understands their role in maintaining accurate and complete documentation for the trial.
There are many reasons why using a quality management software solution like SimplerQMS is better than manual document processes, especially when clinical investigations are concerned.
First of all, it allows you to automate many of the tasks involved in document processing, saving you a lot of time and effort. Additionally, the system keeps track of your documents more effectively, ensuring that they are organized and accessible when you need them, eliminating the need for paper and other physical storage materials.
Furthermore, SimplerQMS provides a secure central repository for all your documents, so you can be confident that they are safe and sound. Its straightforward interface will equip you with a state-of-the-art document control solution with trackable version control that ensures that only the most up-to-date documents are present.
Lastly, and arguably most importantly, clinical research vendors can be granted a special license with controlled access to the SimplerQMS system to help ensure ownership of the data, and streamline documentation processes. This allows your vendor to help keep the data organized and up to date while also simplifying any documentation that needs to be done on their end.
This also adds an extra layer of protection and security to the clinical trial process, giving sponsors more peace of mind.
Final Thoughts
Considering the complexity of today’s world, where you are in one region and your vendors are in a different region of the globe, it becomes essential to have an effective vendor management program in place.
This is not only important in terms of customer satisfaction and enhanced brand value but also a core responsibility since international regulations, guidelines, and standards expect this.
Investing in an efficient Quality Management Solution like SimplerQMS can help streamline vendor management processes and make compliance with current regulatory requirements easier.
To learn more about how SimplerQMS can help your business, book a personalized demo with our experts today and allow us to help you excel in quality management within your organization.

- News & Events

ICH E6 (R2) Requirements for Vendor Oversight
Posted in Process Improvement
Do you have an effective oversight plan? How are you measuring it?
ICH E6 (R1) was modernized to keep pace with the scale and complexity of clinical trials and to ensure appropriate use of technology. It took two decades for that change to be implemented (1996 to 2016) but now the pace of change is happening more rapidly.
ICH E8 (R1) is expected to go into effect in/around June 2020. Like E6 (R2), it calls for identifying factors that are critical to quality at the design stage of the study and planning the study conduct proportionate to the risks of these quality factors. It also calls for consulting with patients and/or patient organizations in the design, planning, and conduct of clinical studies.
The update to E8 will quickly be followed by ICH E6 (R3). The action proposed and endorsed in November 2019, is a full rewrite and reorganization of E6 (R2). It will retain the concept of a proportion risk-based approach and guidance and will expand, modify, and reorganize the sections to provide scientific and ethical guidance. This will enable a diversity of approaches that are relevant and adaptable to a variety of clinical trial designs and innovative technologies.
The common thread across these revisions is a risk-based approach to managing quality.
Section 5 of ICH E6 (R2) requires that sponsors have a system to manage quality. Historically, this was managed by QA, who, very often, did not get involved until there was serious non-compliance at a site or when a sponsor was getting ready for submission and anticipating what sites might get inspected. Now the expectation from the get-go is that quality be managed throughout the life cycle of the study using a risk-based approach focusing on risks to critical processes and data and issues that matter.
When activities are outsourced, including duties and functions sub-contracted to other parties by the CRO, the ultimate responsibility for quality and integrity of trial data always resides with the sponsor who provides oversight. A central lab for critical data (data that are critical to ensure human subject protection and/or reliability of trial results) is one example. Consider a false-positive or false-negative biopsy result and the harm that may pose for a patient – how should that risk be controlled? Identifying risks to the quality of work performed by a CRO and other vendors allows the sponsor to actively manage those risks and reduce the likelihood of severe consequences.
Monitoring is one activity that’s generally outsourced. Risk-based monitoring is one method to control risk. R2 provides flexibility in how you approach monitoring, but you need to justify the rationale for the strategy you have chosen.
FDA guidance on risk-based monitoring recommends periodic review of monitoring reports. Who is going to review those reports? Do they have the required competency? If they don’t, that, too, is a risk to consider.
For effective vendor oversight, consider the following:
- A governance structure to guide and manage the sponsor/CRO relationship. It can vary in complexity depending on the size of the organization. Ideally, you want people with industry experience and knowledge. You should establish processes for communication and escalation and put a charter in place. Set expectations from the start. For example, right-first-time principles.
- A clinical quality agreement . This is just one component of a quality management system. Other elements include policies and procedures, training, issue management and CAPAs, knowledge management, clinical risk management, inspections and audit management, KPIs, and KQIs.
- Documents review . In addition to reviewing study plans and monitoring visit reports, the TMF also needs to be reviewed. By dipping into it periodically, you should be able to see if it is up to date. Both EMA and MHRA expect the TMF to be current and inspection ready. MHRA suggest that you keep an oversight file. Plan ad-hoc effectiveness checks of your oversight plans. What is working and what is not? Modify and improve them as necessary.
- Prevent issues from arising . If you have a well-designed protocol and have sought input from key stakeholders, including patients, hopefully, you will avoid amendments. Seek input from investigators, not just KOLs. Know your patient population and seek input from your CRO. Set your CRO partner up for success.
- Use available technology . Set thresholds, measure KRIs and set quality tolerance limits. This will help ensure the overall quality of trial related processes and will help you identify trends early on so you can take proactive measures.
- Provide oversight commensurate with risk . For example, SUSAR timelines for reporting/ underreporting of SAEs, protocol deviations, CAPAs, etc. Document your oversight.
- Real-time management of risk. Sponsors may plan to adapt the design based on early results. They need data close to real-time to make those decisions. Set expectations at the outset. Monitor performance and provide feedback. Update your plans as necessary and incorporate issues and mitigation activities in ‘lessons learned.’
Good vendor oversight is a combination of tools, processes, and people.
Use the right people at the right sites to give the right dose to the right patient at the right time.
A well-designed protocol with good risk control should result in no rework and no amendments, which ultimately gets products to market sooner.
To learn more about ICH E6 (R2) requirements for vendor oversight, view the recording and slides from our January 2020 webinar.
This website uses cookies to improve functionality and performance. If you continue browsing the site, you are giving implied consent to the use of cookies on this website. See our Privacy Policy for details.
Not a member?
Find out what The Global Health Network can do for you. Register now.
Member Sites A network of members around the world. Join now.
- 1000 Challenge
- ODIN Wastewater Surveillance Project
- CEPI Technical Resources
- UK Overseas Territories Public Health Network
- Global Malaria Research
- Global Snakebite Research
- Global Outbreaks Research
- Sub-Saharan Congenital Anomalies Network
- Global Pathogen Variants
- Global Health Data Science
- AI for Global Health Research
- MRC Clinical Trials Unit at UCL
- Virtual Biorepository
- Rapid Support Team
- The Global Health Network Africa
- The Global Health Network Asia
- The Global Health Network LAC
- The Global Health Network MENA
- Global Health Bioethics
- Global Pandemic Planning
- EPIDEMIC ETHICS
- Global Vector Hub
- Global Health Economics
- LactaHub – Breastfeeding Knowledge
- Global Birth Defects
- Antimicrobial Resistance (AMR)
- Human Infection Studies
- EDCTP Knowledge Hub
- CHAIN Network
- Brain Infections Global
- Research Capacity Network
- Global Research Nurses
- ZIKAlliance
- TDR Fellows
- Global Health Coordinators
- Global Health Laboratories
- Global Health Methodology Research
- Global Health Social Science
- Global Health Trials
- Zika Infection
- Global Musculoskeletal
- Global Pharmacovigilance
- Global Pregnancy CoLab
- INTERGROWTH-21ˢᵗ
- East African Consortium for Clinical Research
- Women in Global Health Research
- Global Health Research Management
- Coronavirus
Research Tools Resources designed to help you.
- Site Finder
- Process Map
- Global Health Training Centre
- Resources Gateway
- Global Health Research Process Map
- About This Site
Downloadable Templates and Tools for Clinical Research
Welcome to global health trials' tools and templates library. please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones have been added. please click on the orange text to download each template., the templates below have been shared by other groups, and are free to use and adapt for your researchstudies. please ensure that you read and adapt them carefully for your own setting, and that you reference global health trials and the global health network when you use them. to share your own templates and sops, or comment on these, please email [email protected]. we look forward to hearing from you.
These templates and tools are ordered by category, so please scroll down to find what you need.
To share your own templates and SOPs, or comment on these, please email [email protected]. We look forward to hearing from you!
- Webinar on community engagement in clinical research involving pregnant women
- Free Webinar: Science, technology and innovation for upskilling knowledge-based economies in Africa
- Open Public Consultation on “Strengthened cooperation against vaccine preventable diseases”
Trial Operations Trial Management Ethics and Informed Consent Resources Trial Design Data Management and Statistics
training
This is Degena Bahrey Tadesse from Tigray, Ethiopia. I am new for this web I am assistant professor in Adult Health Nursing Could you share me the sample/templet research proposal for Global Research Nurses Pump-priming Grants 2023: Research Project Award
I have learned lot..Thanks..
i was wondering why there is no SOP on laboratory procedures ?
Hi, Can you provide me the SOP for electronic signatures in Clinical trial
Do you have an "SOP for Telephonic site selection visit". Kindly Share on my registered mail ID
Thank you for sharing the resources. It is very kind of you.
Hi These tolls are very useful! Thank you
Do you have a task and responsability matrix template for clinical trial managment ? Best
I am very much happy to find myself here as a clinician
Dear Getrude
We have a free 14-module course on research ethics on our training centre; you'll receive a certificate if you complete all the modules and quizzes. You can take it in your own time. Just visit 'Training centre' in the tabs above, then 'short courses'.
Kind regards The Editorial Team
need modules on free online gcp course on research ethics
Estimados: me parece excelente el aporte que han hecho dado que aporta. por un lado a mejorar la transparencia del trabajo como a facilitar el seguimiento y supervisión de los mismos. Muchas gracias por ello
We also have an up to date list of global health events available here: https://globalhealthtrials.tghn.org/community/training-events/
Dear Nazish
Thank you, I am glad you found the seminars and the training courses useful. We list many training events (all relevant to Global Health, and as many of them as possible are either free or subsidised) on the 'community' web pages above. Keep an eye on those for events and activities which you can get involved with. Also, if you post an 'introduction' on the introduction group stating where you are from and your research interests, we can keep you updated of relevant local events.
Thanks so much. These are very helpful seminars. Please let me know any other websites/links that provide free or inexpensive lectures on clinical Research. Appreciate your help.
Hi Nazish, and welcome to the Network. The items here are downloadable templates for you to use; it sounds like you may be seeking lectures and eLearning courses? If so - no problem! You can find free seminars with sound and slides here: https://globalhealthtrainingcentre.tghn.org/webinars/ , and you can find free, certified eLearning courses here: https://globalhealthtrials.tghn.org/elearning . Certificates are awarded for the eLearning courses for those scoring over 80% in the quiz at the end of each course. If you need anything else, do ask! Kind regards The Editorial Team
Hi, I am new to this website and also to the Clinical Research Industry for that matter I only am able to see the PDF of these courses, just wanted to know are these audio lectures and also happen to have audio clips that go with the pdf?
This site is impeccable and very useful for my job!!!!
Thank you for your kind comments.
Fantastic resources
I am delighted you found this website. I earlier introduced it to you because of your prolific interest in health care information and resource sharing....
Please Sign in (or Register ) to view further.
Useful Resources
Related articles.
- PRISMA for Abstracts: Reporting Systematic Reviews in Journal and Conference Abstracts BY Jai K Das
- 5 ways statistics can fool you—Tips for practicing clinicians BY Jai K Das
- How to prepare for a job interview and predict the questions you’ll be asked BY The Editorial Team
- Preparing for and Executing a Randomised Controlled Trial of Podoconiosis Treatment in Northern Ethiopia BY Henok Negussie, Thomas Addissie, Adamu Addissie, Gail Davey
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control BY WHO/ TDR
Most popular tags
- Archive (303)
- archive (103)
- data sharing (70)
- sharing (63)
- training (49)
- malaria (30)
- ACT consortium (25)
- informed consent (7)
- data management (6)
- trial management (6)
- careers (5)
- guidelines (5)
- monitoring (5)
- workshop (5)
- administration (4)
- clinical research (4)
Forms, Tools, & Templates
Filter by Category(ies) Expand/Collapse Category Filter
- Budget/ Finance
- Communication
- Data Management
- OCR Applications
- Project Management
- Protocol Templates
- Recruitment
- Regulatory File/ Investigator Site File
- Source Documentation
- Study Start-Up
- Third Party/ Vendors
- Trial Documents
- Trial Master File
- Regulatory File
0">Matching of items
0 && show_pagination">Page of
There are no matching items
- Forms, Tools, & Templates Description Category(ies) Keyword(s)
- 02.04.02 Investigator's Brochure Addendum Log Track versions of the Investigator’s Brochure Trial Documents TrialDocuments IB
- 01.01.01 Work Instructions TMF - PennBox Instructions explaining training requirements, user roles, access, and use of the TMF in the Veeva Vault system Trial Master File TrialMasterFile Trial Master File
- 01.01.01 Work Instructions TMF - Veeva Instructions explaining training requirements, user roles, access, and use of the TMF in the PennBox system. Trial Master File TrialMasterFile Trial Master File
- 01.02.03 Notice of Disclosure of FCOI Template for written disclosure of FCOI IND; IDE INDIDE Sponsor
- 01.02.03 Statement of Financial Interest - Sponsor Team Member Form to be used by Penn Faculty or staff of the Sponsor team to state presence/absence of financial interests for a particular study. Sponsor Sponsor FCOI, Disclosure, Conflict
- 01.03.01 DSMB Charter Used to clearly describe how the DSMB will function, including frequency of meetings, data to be reviewed, how safety items will be communicated, etc. DSMB DSMB Data safety monitoring board
- 01.03.02 DSMB Contact List Used to compile contact information for the DSMB members and their administrative support staff. May be useful as a reference during trial progress. DSMB DSMB Data safety monitoring board
- 01.03.03 DSMB Meeting Minutes Used to document discussions during DSMB meetings. Includes documentation of attendance and disclosure of financial conflicts. DSMB DSMB Data safety monitoring board
- 01.03.03 DSMB Outcome Email Letter Used to document the outcome of the DSMB meeting to provide to the PI(s). The PI may need this confirmation to provide to their IRB or other local regulatory review committee. DSMB DSMB Data safety monitoring board
- 01.03.03_DSMB_Report Used to provide a summary of trial data to the DSMB. Specific section will differ by study, may be revised based on DSMB request. DSMB DSMB Data safety monitoring board
- 01.03.05 DSMB Qualification Form Used to document potential DSMB member's agreement to serve as part of the DSMB. Includes description of confidentiality and disclosure of financial conflict of interest provisions. DSMB DSMB Data safety monitoring board
- 01.05.01 Correspondence Log Template Log for documenting correspondence including the date, time, participants, and summary of discussion Project Management ProjectManagement Minutes
- 02.01.01 Investigator Brochure Template Guide for developing an Investigator's Brochure Trial Documents TrialDocuments
- 03.01.01 Expanded Access eIND Instructions for submitting an emergency IND request IND IND Emergency use, Investigational New Drug
- 03.01.01 Expanded Access sIND Instructions for submitting a (compassionate use) single patient IND request IND IND Investigational New Drug
- 03.01.01 IDE Application Template Original Investigational Device Exemption (IDE) template in accordance with 21CFR812.20 IDE IDE
- 03.01.01 IND Application Template Template for an application to the FDA for an Investigational New Drug (IND) IND IND
- 03.01.01 Submitting to the FDA This document describes the different way a submission can be sent to the FDA. It applies to clinical research (IND/IDE) and also to Expanded Access of drugs and devices. Sponsor Sponsor Regulatory submissions, CDER, CBER, ESG, CDRH
- 03.01.01 aIDE Application Template Abbreviated Investigational Device Exemption (aIDE) Template IDE IDE
- 03.03.02 IDE Progress Report Template Template for completing an IDE Progress Report IDE IDE Investigational Device Exemption (IDE)
- 03.03.02 IND Annual Report Template Template for completing an IND Annual Report IND IND Investigational New Drug
- 03.03.03 Closing an IND Guide to Sponsor How to discontinue an IND with the FDA. Definitions of the various IND statuses are included below for reference purposes. IND IND Investigational New Drug (IND)
- 03.04.02 Regulatory History Log Template for tracking all regulatory submissions of an IND/IDE IND; IDE INDIDE Sponsor
- 05.02.01 IB Signature Page This document tracks the Principal Investigator's acknowledgement of an Investigator Brochure for their IND study. IND IND Investigator Brochure, Principal Investigator, Investigator New Drug (IND)
- 05.02.02 PI Signature Page The document to be signed by the Principal Investigator of a study at each revision of the study's protocol. Protocol Templates ProtocolTemplates Principal Investigator, Protocol Amendment, Revision
- 05.02.06 Significant Deviation Form This is a form to be used by a Sponsor to document the assessment and impact of deviations associated with clinical trial activities. Sponsor Sponsor Exception
- 05.02.07 PI Qualification Form Template for documenting the qualifications of a Principal Investigator Sponsor Sponsor Principal Investigator (PI)
- 05.02.10 Site Financial Disclosure Form Form utilized by the Sponsor to evaluate financial interests of study investigators. Sponsors must evaluate if a financial conflict of interest (FCOI) exists and if management is required based on this information. Refer to Penn Policy on Conflicts of Interest Related to Research for who meets the definition of “Investigator.” Sponsor Sponsor financial interest, financial conflict of interest
- 05.02.19 IDE Investigator Agreements The investigator agreement of compliance to all requirements of the investigational plan, IDE regulations, and other applicable regulations of the FDA for investigational devices. IDE IDE Statement, Device
- 07.01.01 Work Instructions - Pharmacovigilance Work instructions on sponsor pharmacovigilance (safety) management. This includes a discussion of the sponsor responsibilities related to pharmacovigilance, as well as instructions on conducting the activities, and additional reference resources. Sponsor Sponsor Sponsor, Safety
- 07.02.02 SAE Form Form used to document an SAE that occurs in a trial and to report the relevant information to the sponsor. Trial Documents TrialDocuments Serious Adverse Event (SAE)
- 09.01.01 GLP Qualification Guide Guide to ensuring vendors providing investigational product are qualified and follow Good Laboratory Practices Third Party/ Vendors ThirdPartyVendors
- 09.02.03 Transfer of Obligations Guide to transferring IND/IDE sponsor obligations to another party IND; IDE INDIDE Vendor
- 21 CFR Part 11 for EMR Memo- Penn Medicine Electronic Health Records in Support of Clinical Research Communication; Study Start-Up CommunicationStudyStartUp part 11, 21 cfr part 11, EMR, compliance, pennchart
- CLIA, CAP, Lab References, Lab Director’s CV Email [email protected] to obtain documents Regulatory File/ Investigator Site File; Source Documentation RegulatoryFileInvestigatorSiteFileSourceDocumentation CLIA, CAP, Lab References, lab director, CV
- CRF Testing Script - Blank Template CRF Testing Script - Blank Template Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- CRF Testing Tracker CRF Testing Tracker Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- CRF build and Schedule of Events tracker CRF build and Schedule of Events tracker Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- CRSPR Email Listserve Clinical Research Staff Portal and Registry (CRSPR) is an email listserve to communicate important, timely information to Penn Medicine's Clinical Research Professionals. Needs PennKey login. Sign up by creating a profile, to receive emails from OCR, IRB, CHPS, etc. OCR Applications OCRApplications CRSPR, listserve, application, email, portal, registry
- Case Report Form Amendments Log Track Case Report Form (CRF) versions and changes Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile CRF
- Clinical Research Resource Feasibility Assessment Budget/ Finance BudgetFinance
- Clinical Trial Protocol Template This protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug, biologic, vaccine or device). Protocol Templates ProtocolTemplates
- Close Out Visit Checklist - Investigator Checklist for closing out a protocol Monitoring Monitoring
- Close Out Visit Checklist- Sponsor Investigator Checklist for closing out a protocol by a Sponsor or Sponsor Investigator Monitoring Monitoring
- Closing IDE Guide to Sponsor How to discontinue an IDE with the FDA. Definitions of the various IDE statuses are included below for reference purposes. IDE IDE Investigational Device Exemption (IDE), Closeout
- Cosmos Access Form Complete this form to request access to EPIC/PennChart's COSMOS network for patient data. PennChart access is a pre-requirement. Data Management; Recruitment; Study Start-Up DataManagementRecruitmentStudyStartUp cosmos, patient data, pennchart, data
- Cost Finder Tool with research rates for both Hospital Billing (HB) and Professional Billing (PB), components of an item/service/procedure. Select at least one entity (HUP, PPMC, PAH OR CCH) prior to searching.Pricing may vary by entity. Ability to search by CPT code (preferred) or procedure name. OCR Applications; Study Start-Up OCRApplicationsStudyStartUp Cost Finder, finance, start up
- Data Safety Monitoring Plan (DSMP) With Guidance This template is intended to be used to develop a data safety monitoring plan. Monitoring Monitoring DSMP, monitoring, Data Safety Monitoring Plan
- Database Activation Authorization Database Activation Authorization Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- Database Lockdown Checklist and CRMS Examples Database Lockdown Checklist and CRMS Examples Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crms, source
- Delegation of Responsibility and Signature Log Document study personnel and their role on the study, as delegated by the Principal Investigator. Also serves as a signature log to identify signatures on research forms. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Device Product Information This guidance document assists US IDE, IND, and foreign clinical research device applicant holders in determining what product information is needed and where to place device product information. IDE IDE
- Electronic Data Management Tool This tool is used to assist the determination of what data management tool would be most beneficial for your research study. Budget/ Finance BudgetFinance
- Electronic Database Build and Activation Summary Plan Electronic Database Build and Activation Summary Plan Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation data management , sponsor, source documentation
- GLP Vendor Qualification Form Template to qualify non-clinical vendors Third Party/ Vendors ThirdPartyVendors Good Laboratory Practices
- ICH GCP Essential Documents Table of regulatory files that should be maintained by the PI and Sponsor throughout a clinical research study Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- IND Enabling Nonclinical Safety Study Work Instructions These work instructions are intended as guidance for personnel who (i) conduct or assist in the conduct of Nonclinical Safety Studies (NSS) to be submitted in support of an IND Application or (ii) have oversight of such activities. Although such studies usually occur prior to the submission of an IND, these work instructions also apply to NSS performed after IND approval and in support of clinical development. If an individual is subject to a FINANCIAL CONFLICT OF INTEREST (FCOI) management plan issued by the Vice Provost for Research, the conditions of the management plan will apply to the conduct of NSS. IND IND Investigational New Drug (IND)
- IND IDE Exemption Amendment Risk Assessment This form provides instructions, and may be used to document, the Sponsor Investigator’s risk assessment for minor changes to a protocol that has previously received an IND or IDE exemption determination. IND; IDE INDIDE Modification
- IND-IDE Sponsor Responsibilities Guide Responsibilities of an IND/IDE Sponsor IND; IDE INDIDE Investigational New Drug (IND), Investigational Device Exemption (IDE)
- Informed Consent Form (ICF) Version Log Tracking log of approvals for Informed Consent Form Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Investigational Product Accountability Study Log Study-level investigational product accountability log (all subjects on one log) Sponsor Sponsor
- Investigational Product Accountability Subject Log Subject specific investigational product accountability log Sponsor Sponsor
- Memo from Penn IRB Memo from Penn IRB regarding the IRB not disclosing names of its members. This is a necessary component of the IDE application. IDE IDE Institutional Review Board (IRB)
- Monitoring Analysis Template This tool is intended to assist in the planning for monitoring a clinical trial. Monitoring Monitoring
- Monitoring Assessment Guidance Document This document is designed to assist study teams with completing an appropriate monitoring summary for the Penn IRB at the time of continuing review. Monitoring Monitoring
- Monitoring Close Out Visit Template Monitoring visit report template for the conduct of a close out monitoring visit at the end of a clinical trial. Monitoring; Sponsor; Trial Master File MonitoringSponsorTrialMasterFile
- Monitoring Findings Template Monitoring Findings Template Monitoring Monitoring findings, monitoring, site
- Monitoring Visit Report - Sponsor Summary Template for reporting monitoring findings to Sponsor Monitoring Monitoring
- Monitoring Visit Tracking Log Log used to document monitoring visits over the course of a trial Monitoring Monitoring
- Note to File Template To be used to create a Note to File which are written to identify a discrepancy or problem in the conduct of the clinical research study. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- On-Site Query Report Form Worksheet for documenting the identification and resolution of queries Monitoring Monitoring
- Participant Visit Schedule Tool for tracking the proposed and actual dates for subject study visits Study Start-Up StudyStartUp
- PennCRMS CRF Guidelines and Tips PennCRMS CRF Guidelines and Tips Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- PennChart - Patient Registration Form EMPI If you need your patient/subject setup in PennChart within 24 hours Budget/ Finance BudgetFinance
- Pre-Screen Phone Script (Incoming Call) Pre-screen phone script template for incoming calls from potential research participants IND; Recruitment; Regulatory File/ Investigator Site File INDRecruitmentRegulatoryFileInvestigatorSiteFile
- Pre-Screen Phone Script (Outgoing Call) Pre-screen phone script template for outgoing calls to potential research participants Recruitment; Regulatory File/ Investigator Site File RecruitmentRegulatoryFileInvestigatorSiteFile
- Principal Investigator (PI) Qualification Form Template for documenting the qualifications of a Principal Investigator Third Party/ Vendors ThirdPartyVendors Principal Investigator (PI); Qualification
- Principal Investigator Compliance Assessment (PICA) The Principal Investigator Compliance Assessment (PICA) is a tool which can be used to monitor or assess the overall conduct of a study. This document is required for all active, high risk studies conducted at Penn. Monitoring Monitoring
- Prospective Reimbursement Analysis/ Medicare Coverage Analysis - Budget Template Template for completing the prospective reimbursement analysis/ medicare coverage analysis and industry sponsored clinical research budget. Budget/ Finance; Regulatory File/ Investigator Site File BudgetFinanceRegulatoryFileInvestigatorSiteFile
- Prospective Study Design with no Investigational Product (IP) Template Use this template to develop a clinical research protocol that does not involve an investigational product. E.g. Comparative effectiveness study, a cohort design, case control study, etc. Protocol Templates ProtocolTemplates
- Protocol Adverse Event Log A Log for recording adverse events Regulatory File RegulatoryFile Adverse Event, Adverse, Event, Log
- Protocol Amendments Log Track versions of the IRB approved Protocol Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Protocol Deviation Log To track all protocol deviations. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Protocol Training Log Log for documenting the training of research personnel on the research protocol Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- RBA Business Admin Approval Tipsheet Tipsheet for the RBA. Shows how to route request for RBN to your BA and OCR Finance for RBN creation Budget/ Finance; OCR Applications; Project Management; Study Start-Up BudgetFinanceOCRApplicationsProjectManagementStudyStartUp RBA, RBN, Research Billing application, Research Billing number, finance, BA
- RBA New Request Tipsheet Tipsheet with steps on how to submit a new request for a Research Billing Number in the Research Billing Application Budget/ Finance; OCR Applications; Project Management; Study Start-Up BudgetFinanceOCRApplicationsProjectManagementStudyStartUp RBA, Research Billing Application, Finance, RBN, billing number
- Recording, Assessment, and Reporting of Deviations This document aims to help teams with the recording of deviations and exceptions of an approved protocol, and the reporting requirements to the Penn IRB and Sponsor IND; IDE; Regulatory File/ Investigator Site File; Sponsor INDIDERegulatoryFileInvestigatorSiteFileSponsor
- Recruitment Letter - Physician to Established Patient Template for recruitment letter from an external, referring physician to his/her patient about a research study Recruitment Recruitment
- Recruitment Letter - Physician to Physician Letter Template Template for recruitment letter from one physician to another about a research study Recruitment Recruitment
- Recruitment Letter – PI to Patient Template Template for recruitment letter from the Principal Investigator to his/her patient about a research study Recruitment Recruitment
- Recruitment Letter- Physician to Unknown Potential Subject Template for recruitment letter from a physician to a potential research subject about a research study Recruitment Recruitment
- Reportable Event Form (Electronic) Form for capturing the details of a Reportable Event for informing a sponsor, manufacturer, or other reporting entity Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Reportable Event Form (Paper) Form for capturing the details of a Reportable Event for informing a sponsor, manufacturer, or other reporting entity Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Research Billing Application (RBA) All studies utilizing UPHS services/procedures and/or Imaging Core/Service Center(s) will need to be registered in the Research Billing Application (RBA), regardless of the payor (insurance or research grant). A Research Billing Number (RBN) will be generated for those studies where all or a portion of visits/tests/procedures associated with the research protocol are being billed to the research grant. OCR Applications OCRApplications RBA, billing, research billing application, hospital, services, grant, payor, insurance
- Residual Balance Transfer Request Form Form used to request the residual balance to be transferred from appropriate contracts Budget/ Finance BudgetFinance
- Retrospective Study Protocol Template This protocol template is designed to facilitate the creation of a retrospective clinical research protocol. Protocol Templates ProtocolTemplates
- Risk Assessment Template for performing a clinical trial risk assessment for monitoring Monitoring Monitoring
- Screening and Enrollment Log Document subjects who have been screened and/or enrolled Recruitment; Regulatory File/ Investigator Site File RecruitmentRegulatoryFileInvestigatorSiteFile
- Site Guidance for Source Documentation This document contains information about Source Documentation for clinical trials. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Site Initiation Visit Checklist Checklist for conducting a Site Initiation Visit IND; IDE; Sponsor INDIDESponsor
- Site Qualification Report Tool for documenting the review and qualification assessment of a site and principal investigator Monitoring; Regulatory File/ Investigator Site File MonitoringRegulatoryFileInvestigatorSiteFile
- Site Visit Log Document the dates of Monitoring Visits Monitoring Monitoring
- Specimen Preparation Checklist Instructions for collecting, labeling and processing specimens for shipment Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Specimen Shipping Log Log the collection and shipment of specimens Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Sponsor - IDE Investigator List Template for the submission to the FDA-CDHR of the semi-annual list of investigators required by 21CFR 812.150 IDE IDE Investigational Device Exemption (IDE)
- Sponsor Monitoring Plan Guide Guide for developing an IND/IDE sponsor's monitoring plan IND; IDE; Sponsor INDIDESponsor
- Sponsor Welcome Letter Template for use in negotiating with an industry sponsor. Budget/ Finance BudgetFinance
- Study Admin File_Device Template for developing a study administration file or regulatory binder for a device trial Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Study Admin File_Drug Template for developing a study administration file or regulatory binder for a drug trial Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Study Admin File_Social Behavioral Template for developing a study administration file or regulatory binder for a social behavioral study Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Study Feasibility Assessment Tool for assessing the resources, recruitment potential, and logistical considerations of a particular study Study Start-Up StudyStartUp
- Subject Contact Information Sheet Collect contact information for research subjects Source Documentation SourceDocumentation
- Subject Eligibility Checklist Template for documenting review of Inclusion and Exclusion criteria Source Documentation SourceDocumentation
- Testing CRFs Overview in PennCRMS Testing CRFs Overview in PennCRMS Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- Trial Master File Template for a Trial Master File Trial Master File TrialMasterFile Trial Master File (TMF), Sponsor
- Understanding Using an EDC - PennCRMS W Understanding Using an EDC - PennCRMS Workflows Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, crms, sponsor, source, crf
- W-9 IRS Form (No Form) Federal form used to report income paid to an individual Budget/ Finance BudgetFinance
- Zone 2 Core Document Change Control Sponsor checklist to document steps taken for an operationally compliant revision of core documents Sponsor Sponsor Protocol, Informed Consent Form (ICF), Investigator Brochure (IB)


Vendor Management and Oversight of Clinical Trials
Proper oversight is critical to ensuring patient safety, as well as mitigating the risks and financial implications of costly change orders, trial delays, non-productive clinical sites, rework (e.g. amendments), and data quality issues.
- Qualifying and selecting the right vendors for your program
- Assessing risk and developing a vendor risk management plan
- Designing and executing against an effective oversight plan
Download the white paper today
Who should watch, ceu credits, certificate of attendance.
DATE Time: 1 p.m. ET, 12 p.m. CT, 10 a.m. PT Duration: 1 hour
Download Now
Please enable Javascript to view this form.

Guest Column | October 18, 2018
A better approach to selecting and overseeing gcp/glp vendors and processes.
By Judy Carmody , Ph.D., Carmody Quality Solutions, LLC

The second article discussed pitfalls to avoid when developing GCP SOPs. This piece outlines the best approach to develop systems for selecting and monitoring vendors to ensure a high-quality study with data integrity — and minimal patient risk.
New therapy development is a long and complex process, and more sponsors are outsourcing clinical trial activities. Yet multiple reports from GCP regulatory inspections found major issues related to monitoring activities and data management, with CROs and sponsors accounting for nearly 43 percent of the total findings . To address these concerns, the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) released an amended version of the international guidelines for GCP: ICH GCP E6(R2).
The fundamental differences between the R1 and R2 versions of the guidelines relate to sponsor/investigator responsibilities and risk-based monitoring. In essence, the new regulations address concerns and questions about whether sponsors and CROs are taking enough care when assessing:
- how sponsors select and monitor vendors
- the execution process of a clinical trial
- data integrity, that is whether data submitted in the dossier is credible and accurate.
This article series has focused on how quality can collaborate with GCP and GMP groups to improve ICH E6(R2) compliance. However, in the case of vendor selection and oversight SOPs, I recommend separating GCP/GLP processes from GMP processes.
Although we think of it in a linear fashion, the process of vendor selection and qualification is not a linear process. The two can happen in parallel, with flexibility and required compliance and oversight. Steps can happen in parallel to facilitate and expedite the vendor selection process — and SOPs can be written in such a manner. You can move forward with a low amount of risk and build in flexibility that allows you to remain in compliance and avoid bottlenecks.
The flexibility comes from precise and clear SOPs. This allows each group to focus on specifics rather than vague, lumped-together generalities that can create inefficiencies, redundancies, training burdens, and “paralysis analysis” when attempting to follow long, complex SOPs. For GCP SOPs, one main issue that is different from GMP is GCP is responsible for the “transfer of obligations” (TOO) to the vendor.
Many companies combine the vendor selection process for GMP/GLP/GCP into one document, but they are all quite different processes. When grouped together, these processes become ripe for examination and inspection from regulatory bodies. As a result, we are seeing a heightened priority and urgency from the FDA to require clarity around the specifics of GCP procedures, such as TOO.
To improve the system to select and oversee vendors and processes, start with the following SOPs that separate GLP/GCP and GMP:
- Vendor selection SOP for vendors supporting GCP/GLP activities: This SOP includes clear expectations and requirements of the relationship with the vendor, which include the TOO that will be defined as part of the vendor contract.
- Vendor selection SOP for vendors supporting GMP activities: This SOP focuses on product and facility specifics.
- Vendor qualification SOP, which is driven by the sponsor’s quality department: This SOP outlines how to create and maintain the clinical audit plan (i.e., the processes for planning, conducting, and reporting clinical GCP vendor audits) to ensure reliability of data and the protection of subjects’ rights/safety. Quality owns the list of vendors and/or qualifies vendors if they are not on the list.
- Vendor oversight plan SOP, which describes the process for sponsor oversight of GCP/GLP vendors: This SOP describes the development, approval, and management of the vendor oversight plan used to manage clinical trial vendors. This ensures CROs and clinical trial vendors perform their trial responsibilities in a manner consistent with standards set by the sponsor, as well as GCP and the FDA and other relevant regulatory bodies.
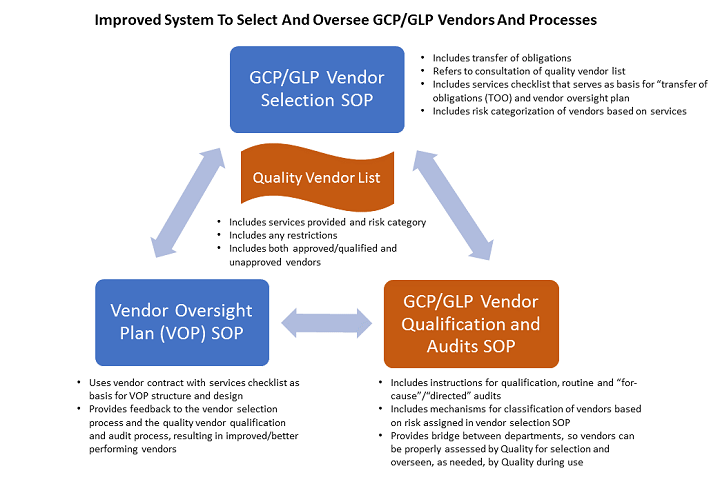
The vendor selection SOPs (#1 and #2 noted above) are similar in flow but contain different elements that justify separate processes. They begin with identifying the right people to bring to the table and completing a services checklist that provides the basis for constructing the RFP. The services checklist for GCP vendors is different than the checklist for GMP vendors since the services required of each are different.
Both selection processes refer to a vendor list, which quality owns and maintains, to determine if the vendor finalist(s) are quality approved/qualified vendors for the desired services. If not, the processes contain instructions for notifying quality of the intent to use a new vendor or a current vendor for new services and reference the associated vendor qualification SOPs. In this fashion, the vendor selection and qualification SOPs are linked together, which creates a more streamlined and collaborative approach.
The vendor qualification SOP (#3) is owned by quality assurance and provides instruction for categorization of each vendor type, based on risk with the associated criteria a vendor must meet to achieve a qualified status. If an audit is required, the timing for performing a requalification audit is also provided. Once the vendor is assessed by quality, the vendor list is updated, and quality notifies the stakeholders of the outcome. Again, quality is the link between the two processes and the GCP/GLP and GMP groups, improving process efficiency.
Based on the outcome of quality’s assessment, the vendor is selected, and the contract is negotiated. The vendor selection SOP instructs you to attach the completed services checklist to the contract as an appendix, since it outlines the TOO. Since the TOO is now part of the contract, you eliminate rounds of review and inspection associated with rechecking the services checklist and then going through the TOO again. It is all becomes part of the contract.
To then create the vendor oversight plan (#4), use the vendor contract(s) with the associated services checklist as a starting point. There are different approaches in designing a vendor oversight plan, but I recommend having a study oversight plan that outlines sponsor oversight of the operational activities outsourced to a CRO, and then creating separate appendices for other clinical development outsourced activities, which may not be assumed by the CRO (e.g., pharmacovigilance, translational medicine, and clinical pharmacology). With this structure, modifications can be made to each section without having to revise the entire plan, which makes the process more efficient and just as compliant.
Another important element of the vendor oversight plan is to identify which SOPs (sponsor or vendor) will be followed. Each section of the plan should include this in addition to the issues escalation plan.
Overall, the goal is to develop a seamless process with natural trigger points that involve quality personnel. When you change services with a provider, you go back to the contract and adjust these services, which should trigger both a consultation of the vendor list and possible notification to quality for assessment/qualification, and a revision to the vendor oversight plan to adjust oversight activities for the changed services.
The approach of separating GCP/GLP and GMP in vendor selection and oversight SOPs can lead to streamlined and efficient processes, which allow for better oversight, data integrity, and overall patient safety.
About the Author

Carmody founded Avatar Pharmaceutical Services, an FDA-registered contract research organization that provided quality, submission-ready, customized analytical services in compliance with cGMP. She grew Avatar to 25+ employees and more than 75 clients before it was purchased by a Boston-based pharmaceutical company in 2010. She holds a Ph.D. in analytical chemistry from Clark University in Worcester, Massachusetts. Please connect with her on LinkedIn .
Like what you are reading?
Sign up for our free newsletter, newsletter signup.


- Clinical Research 70
- Clinical Data Management 42
- Pharmacovigilance 51
- Medical Writing 57
- Regulatory Affairs 42
- Getting Started 6
- Troubleshooting 5
Clinical Research Vendor Management
Clinical research vendor management involves the ongoing oversight, coordination, and monitoring of vendor activities to ensure their effective performance and adherence to project requirements. It encompasses various aspects, including vendor relationship management, performance evaluation, issue resolution, and contract and financial management. Here are some key considerations in clinical research vendor management:
- Vendor Relationship Management: Foster effective communication and collaboration with vendors to establish a positive working relationship. Regularly engage with vendor representatives to discuss project updates, address concerns, and ensure alignment on project goals. Maintain open lines of communication to promote transparency and address issues proactively.
- Performance Monitoring and Evaluation: Regularly assess vendor performance against predefined key performance indicators (KPIs) and metrics. This may include evaluating factors such as data quality, adherence to timelines, compliance with regulatory requirements, and overall service delivery. Use performance metrics to identify areas of improvement and provide constructive feedback to vendors.
- Issue and Risk Management: Monitor vendor performance and promptly address any issues or risks that arise during the course of the project. Establish a clear escalation process to address critical issues and ensure timely resolution. Collaborate with vendors to develop mitigation strategies for identified risks and implement corrective actions as necessary.
- Contract and Financial Management: Maintain accurate and up-to-date vendor contracts, including clearly defined deliverables, timelines, pricing, and payment terms. Regularly review contract compliance and manage any necessary contract amendments or renewals. Monitor vendor invoices, ensure accuracy, and process payments in accordance with agreed-upon terms.
- Change Management: Communicate any changes to project requirements or scope to vendors and manage the associated impact on their deliverables and timelines. Collaborate with vendors to assess the feasibility of requested changes, negotiate any necessary modifications to the contract, and ensure smooth implementation of changes without compromising the project’s objectives.
- Vendor Oversight and Audit: Conduct periodic audits or site visits to monitor the vendor’s compliance with regulatory requirements, quality systems, and standard operating procedures. Review documentation, processes, and facilities to ensure adherence to industry standards. Provide feedback and recommendations for improvement, as needed.
- Performance Reviews and Lessons Learned: Conduct regular performance reviews with vendors to assess their overall performance and identify areas for improvement. Engage in open discussions to share lessons learned and best practices. Use these reviews as an opportunity to strengthen the partnership and identify strategies to enhance future collaborations.
- Relationship with Internal Stakeholders: Maintain close collaboration with internal stakeholders, such as clinical teams, data managers, and project sponsors, to ensure alignment with vendor activities. Facilitate effective communication between internal teams and vendors to address any challenges, resolve issues, and maintain project momentum.
Effective clinical research vendor management requires proactive engagement, clear communication, and strong project oversight. By fostering a collaborative and transparent relationship with vendors, addressing issues in a timely manner, and regularly evaluating their performance, you can optimize vendor contributions and ensure successful project outcomes.
You may be interested in the programs below:

Introduction to Clinical Research

Ethics in Clinical Research

Diploma in Clinical Research
Advertisement
Toolkit for ICH E6 (R2) Quality Risk Management for Small to Medium Size Companies
- Research Article
- Published: 02 January 2020
- Volume 54 , pages 900–921, ( 2020 )
Cite this article

- Andrew Della-Coletta 1 ,
- Terry Katz 2 ,
- Kathy Kupka 3 ,
- Sandy Mohan 4 ,
- Kamila A. Novak 5 ,
- Maryrose Petrizzo 6 &
- Sarah Ann Silvers 7
990 Accesses
5 Citations
7 Altmetric
Explore all metrics
One of the most significant revisions to the ICH E6 GCP Guideline in the last 20 years was issued in November 2016, adopted by the EMA in December 2016 and by the FDA as a Guidance Document in March 2018. The new section on Quality Management requires the implementation of a systematic approach for managing risks throughout the course of a clinical study. The addendum also emphasizes appropriate sponsor oversight. Currently available risk management solutions are fairly elaborate, having been developed for and adopted mainly by larger companies. Small to medium size companies find these solutions too complex and not easily adaptable. In this paper, we present a simple but robust toolkit for clinical study risk management for small to medium sized organizations in order to facilitate compliance. The toolkit consists of customizable templates for the following: Clinical Risk Management SOP; Clinical Risk Management Plan; Vendor Oversight SOP; Vendor Oversight Plan; and Clinical Risk Log. The tools were prepared by the DIA GCP-QA Community members and presented at the 2018 DIA Annual Meeting in Boston.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Comprehensive Assessment of Risk-Based Quality Management Adoption in Clinical Trials
Risk-based monitoring (rbm) implementation: challenges and potential solutions, quality risk management framework: guidance for successful implementation of risk management in clinical development.
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), Integrated Addendum to ICH E6(r1): Guideline for Good Clinical Practice E6(r2), EMA/CHMP/ICH/135/1995. https://www.ich.org/products/guidelines/efficacy/efficacy-single/article/integrated-addendum-good-clinical-practice.html (2016). Accessed 27 Mar. 2019.
U.S. Department of Health and Human Services Food and Drug Administration, E6 (R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1) Guidance for Industry ICH E6. https://www.fda.gov/downloads/Drugs/Guidances/UCM464506.pdf (2018). Accessed 27 Mar 2019.
European Clinical Research Infrastructures Network Integrating Activity, Guideline on Risk Management for Clinical Research, Version 1.0, ECRIN-IA CP-CSA_INFRA-2011-1.1.5 (Facilities and resources for multinational clinical trials). https://ssl2.isped.u-bordeaux2.fr/OPTIMON/docs/citations/2015-02-16-Guideline%20on%20risk%20management%20for%20clinical%20research_1.0x.pdf . Accessed 27 Mar. 2019.
MRC/DH/MHRA Joint Project, Risk-adapted Approaches to the Management of Clinical Trials of Investigational Medicinal Products, Version: 10th October 2011. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/343677/Risk_adapted_approaches_to_the_management_of_clinical_trials_of_investigational_medicinal_products.pdf . Accessed 27 Mar 2019.
International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use ICH, Harmonised Tripartite Guideline, Quality Risk Management Q9, Annex 1: Risk Management Methods and Tools. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf . Accessed 27 Mar 2019.
International Organization for Standardization, ISO 21500:2012, Guidance on Project Management. https://www.iso.org/standard/50003.html . Accessed 27 Mar 2019.
World Medical Association Declaration Of Helsinki – Ethical Principles For Medical Research Involving Human Subjects. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ . Accessed 30 Mar 2019.
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), Integrated Addendum to ICH E6 (R2). https://www.ich.org/products/guidelines/efficacy/efficacy-single/article/integrated-addendum-good-clinical-practice.html . Accessed 27 Mar. 2019.
U.S. Department of Health and Human Services Food and Drug Administration, E6 (R2) Good Clinical Practice: Integrated Addendum to ICH E6 (R1) Guidance for Industry ICH E6. https://www.fda.gov/downloads/Drugs/Guidances/UCM464506.pdf (2018). Accessed 27 Mar 2019.
General Data Protection Regulation, Regulation (EU) 2016/679 of the European Parliament and of the Council. https://ec.europa.eu/commission/priorities/justice-and-fundamental-rights/data-protection/2018-reform-eu-data-protection-rules_en . Accessed 28 Mar 2019.
Munro RA, Maio MJ, Nawaz MB, Ramu G, Zrymiak DJ. Failure mode and effects analysis (FMEA), the certified six sigma green belt handbook. 2nd ed. Milwaukee, WI: ASQ Quality Press; 2008. p. 100–7.
Google Scholar
U.S. Department of Health and Human Services Food and Drug Administration, E6 (R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1) Guidance for Industry ICH E6. https://www.fda.gov/downloads/Drugs/Guidances/UCM464506.pdf (2018). Accessed 27 Mar 2019. U.S. Department of Health and Human Services, Food and Drug Administration, Guidance for Industry: Q9 Quality Risk Management. https://www.fda.gov/downloads/Drugs/Guidances/ucm073511.pdf . Accessed 27 Mar 2019.
Download references
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and affiliations.
DC Life Sciences Group, Cary, NC, USA
Andrew Della-Coletta
Merck Animal Health (A Subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA), Madison, NJ, USA
CompleWare Corporation, Iowa City, IA, USA
Kathy Kupka
Immune Design Corp. (A Subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA), 601 Gateway Blvd, Ste 250, South San Francisco, CA, 94080, USA
Sandy Mohan
KAN Consulting, Athens, Greece
Kamila A. Novak
Clinical Quality Assured LLC, Wilmington, DE, USA
Maryrose Petrizzo
CE3, Inc, Guilford, CT, USA
Sarah Ann Silvers
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sandy Mohan .
Ethics declarations
Conflict of interest.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (XLSX 42 kb)
Rights and permissions.
Reprints and permissions
About this article
Della-Coletta, A., Katz, T., Kupka, K. et al. Toolkit for ICH E6 (R2) Quality Risk Management for Small to Medium Size Companies. Ther Innov Regul Sci 54 , 900–921 (2020). https://doi.org/10.1007/s43441-019-00004-6
Download citation
Received : 23 May 2019
Accepted : 23 August 2019
Published : 02 January 2020
Issue Date : July 2020
DOI : https://doi.org/10.1007/s43441-019-00004-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- ICH E6 (R2)
- Quality risk management
- Vendor oversight
- Small companies
- Medium companies
- Find a journal
- Publish with us
- Track your research

COMMENTS
Initiating Statement of Work (SOW), Due Diligence, and Service Level Agreements (SLAs) 1. Conduct a Request for Information (RFI) Assessment. The Request for Information (RFI) process is integral to clinical trial vendor selection for every life science company willing to conduct trial-based investigational activities.
Learn how to manage quality and integrity of trial data outsourced to CROs using a risk-based approach. This web page provides guidance on governance, expectations, documents review, effectiveness checks, and technology for vendor oversight.
Data Management : Data handling study team agreement. Data Recording SOP. Data clarification form : Data management plan : CRF template -generic malaria : CRF template generic : CRF tracking template : Data Collection : CRF template -generic malaria. Data Recording SOP. CRF template generic. Blood Sampling SOP. CRF tracking template : Study and ...
Contact Us Penn Medicine Clinical Research. 8041 Maloney Building 3400 Spruce Street Philadelphia, PA 19104-4238 . 215-662-4484 Email Us
V endor Selection and Management. Sachi Amatya * and Dawn Edgerton. Vendor s provide services that are critical to the success ful outcome of a clinical study, yet sponsors. retain the ultimat e ...
Vendor Management and Oversight of Clinical Trials. Proper oversight is critical to ensuring patient safety, as well as mitigating the risks and financial implications of costly change orders, trial delays, non-productive clinical sites, rework (e.g. amendments), and data quality issues. Download this white paper for practical insights and ...
optimized outcomes. This article serves as a guide to understanding the significance and intricacies of vendor management in the realm of clinical research. Keywords: Clinical trials; Vendor management; Vendor. technology providers, and other specialized service providers. Clinical trials are complex endeavors that require expertise and
Vendor oversight plan SOP, which describes the process for sponsor oversight of GCP/GLP vendors: This SOP describes the development, approval, and management of the vendor oversight plan used to manage clinical trial vendors. This ensures CROs and clinical trial vendors perform their trial responsibilities in a manner consistent with standards ...
Updated June 5, 2023. Clinical research vendor management involves the ongoing oversight, coordination, and monitoring of vendor activities to ensure their effective performance and adherence to project requirements. It encompasses various aspects, including vendor relationship management, performance evaluation, issue resolution, and contract ...
Clinical risk management plan. 3. Vendor oversight SOP. 4. Vendor oversight plan. 5. Clinical risk log. Clinical Risk Management SOP. This SOP template provides a framework for risk identification, classification, monitoring, and mitigation throughout the life cycle of a clinical trial as well as evaluation of process effectiveness. Clinical ...