- Structure of Atom
- Oil Drop Experiment

Milliken's Oil Drop Experiment
The Millikens Oil Drop Experiment was an experiment performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the charge of an electron. This experiment proved to be very crucial in the physics community.
Millikens Oil Drop Experiment Definition
In the experiment, Milliken allowed charged tiny oil droplets to pass through a hole into an electric field. By varying the strength of the electric field the charge over an oil droplet was calculated, which always came as an integral value of ‘e.’
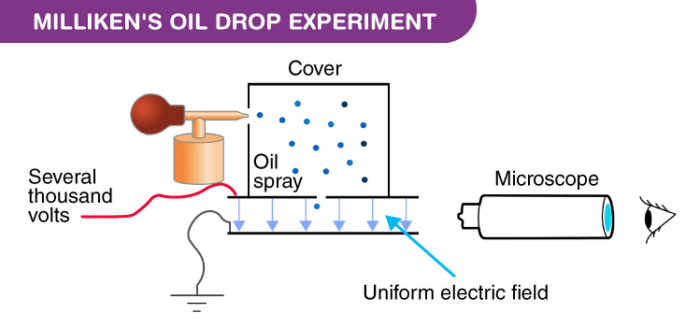
Apparatus of the Milliken’s Oil Drop Experiment
The apparatus for the experiment was constructed by Milliken and Fletcher. It incorporated two metal plates held at a distance by an insulated rod. There were four holes in the plate, out of which three were there to allow light to pass through them and one was there to allow viewing through the microscope.
Ordinary oil wasn’t used for the experiment as it would evaporate by the heat of the light and so could cause an error in the Millikens Oil Drop Experiment. So, the oil that is generally used in a vacuum apparatus which is of low vapour pressure was used.
Milliken’s Oil Drop Experiment Procedure
- Oil is passed through the atomizer from where it came in the form of tiny droplets. They pass the droplets through the holes present in the upper plate of the apparatus.
- The downward motions of droplets are observed through a microscope and the mass of oil droplets, then measure their terminal velocity.
- The air inside the chamber is ionized by passing a beam of X-rays through it. The electrical charge on these oil droplets is acquired by collisions with gaseous ions produced by ionization of air.
- The electric field is set up between the two plates and so the motion of charged oil droplets can be affected by the electric field.
- Gravity attracts the oil in a downward direction and the electric field pushes the charge upward. The strength of the electric field is regulated so that the oil droplet reaches an equilibrium position with gravity.
- The charge over the droplet is calculated at equilibrium, which is dependent on the strength of the electric field and mass of droplet.
Milliken’s Oil Drop Experiment Calculation
F up = F down
F up = Q . E
F down = m.g
Q is an electron’s charge, E is the electric field, m is the droplet’s mass, and g is gravity.
One can see how an electron charge is measured by Millikan. Millikan found that all drops had charges that were 1.6x 10 -19 C multiples.
Milliken’s Oil Drop Experiment Conclusion
The charge over any oil droplet is always an integral value of e (1.6 x 10 -19 ). Hence, the conclusion of Millikens Oil Drop Experiment is that the charge is said to be quantized, i.e. the charge on any particle will always be an integral multiple of e.
Frequently Asked Questions – FAQs
What did millikan’s oil drop experiment measure.
Millikan oil-drop test, the first simple and persuasive electrical charge calculation of a single electron. It was first conducted by the American physicist Robert A. in 1909. He discovered that all the drops had charges that were simple multiples of a single integer, the electron’s fundamental charge.
What is the importance of Millikan’s oil drop experiment?
The experiment with Millikan is important since it defined the charge on an electron. Millikan used a very basic, very simple system in which the behaviour of gravitational, electrical, and (air) drag forces were controlled.
What did Millikan conclude after performing his oil drop experiment?
An integral multiple of the charge on an electron is the charge on every oil decrease. About an electric force. In a relatively small amount, the charge and mass of the atom must be condensed.
Why charges are quantized?
Charges are quantized since every object’s charge (ion, atom, etc.) Charge quantization, therefore, implies that no random values can be taken from the charge, but only values that are integral multiples of the fundamental charge (proton / electron charge).
Can charge be created or destroyed?
The Charge Conservation Law does not suggest that it is difficult to generate or remove electrical charges. It also means that any time a negative electrical charge is produced, it is important to produce an equal amount of positive electrical charge at the same time so that a system’s overall charge does not shift.
For more information about quantum physics , download BYJU’S-The learning app to play store and app store.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- Why Does Water Expand When It Freezes
- Gold Foil Experiment
- Faraday Cage
Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks
Who Did the Oil Drop Experiment?
The Oil Drop Experiment was performed by the American physicist Robert A Millikan in 1909 to measure the electric charge carried by an electron . Their original experiment, or any modifications thereof to reach the same goal, are termed as oil drop experiments, in general.
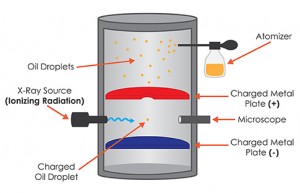
What is the Oil Drop Experiment?
In the original version, Millikan and one of his graduate students, Harvey Fletcher, took a pair of parallel horizontal metallic plates. A uniform electric field was created in the intermediate space by applying a potential difference between them. The plates were held apart by a ring of insulating material. The ring had four holes, three for allowing light to illuminate the setup, and the fourth one enabled a microscope for viewing. A closed chamber with transparent walls was fitted above the plates.
At the beginning of the experiment, a fine mist of oil droplets was sprayed into the chamber. In modern setups, an atomizer replaces the oil droplets. The oil was so chosen such that it had a low vapor pressure and capable of charging. Some of the oil drops became electrically charged by friction as they forced their way out of the nozzle. Alternatively, charging could also be induced by incorporating a source of ionizing radiation , such as an X-Ray tube, in the apparatus. The droplets entered the space between the plates and raised or fell, according to the requirement, by varying plate voltage.
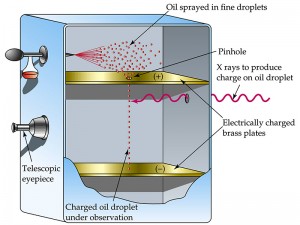
In terms of the present-day arrangement, when the electric field is turned off, the oil drops fall between the plates under the action of gravity only. The friction with the oil molecules in the chamber makes them reach their terminal velocity fast. The terminal velocity is the constant speed that a freely falling object eventually reaches when the resistance of the medium through which it is falling prevents further acceleration . Once the field is turned on, the charged drops start to rise. This motion happens since the electric force directed upwards is stronger than the gravitational force acting downwards. One charged drop is selected and kept at the center of the field of view of the microscope after allowing all other drops to fall by alternately switching off the voltage source. The experiment is conducted with this drop.

Theory and Calculations
First, the oil drop is allowed to fall in the absence of an electric field, and its terminal velocity, say v 1 , is found out. Using Stokes’ law, the drag force acting on the drop is calculated using the following formula.
Here r is the radius of the drop and ɳ, the viscosity of air.
The weight of the drop, w’, which is the product of its mass and acceleration due to gravity g, is given by the equation,
where ρ is the density of the oil.
However, what we need here is the apparent weight w of the drop in the air given by the difference of the actual weight and the upthrust of the air. We can express w by the following formula.
Here ρ air denotes the density of air.
When the drop attains terminal velocity, then it has no acceleration. Hence, the total force acting on it must be zero. That means,
The above equation can be used to find out the value of r. Once r is calculated, the value of w can easily be found out from equation (i) marked above.
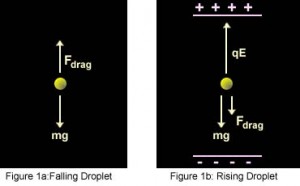
Now after turning on the electric field between the plates, the electric force F E acting on the drop is,
Where E is the electric field and q the charge on the oil drop. For parallel plates, the formula for E is,
Here V is the potential difference and d the distance between the plates. That implies,
Now if we adjust V to make the oil drop remain steady at a point, then
Thus, the value of q can be calculated. By repeatedly applying this method to multiple oil droplets, the electric charge values on individual drops were always found to be integer multiples of the smallest value. This lowest charge could be nothing but the charge on the elementary particle, electron. By this method, the electronic charge was calculated to be approximate, 1.5924×10 −19 C, making an error of 1% of the currently accepted value, 1.602176487×10 −19 C. All subsequent research pointed to the same value of charge on the fundamental particle.
Millikan was able to measure both the amount of electric force and magnitude of electric field on the tiny charge of an isolated oil droplet and from the data determine the magnitude of the charge itself. Millikan’s oil drop experiment proved that the electric charge is quantized in nature. The electric charge appears in quanta of magnitude 1.6 X 10 -19 C in oil droplets.
Robert Millikan’s Oil Drop Experiment Animation
Millikan’s oil drop experiment and the atomic theory.
Until the time of the Oil Drop Experiment, the world had little or no knowledge of what is present inside an atom . Earlier experiments by the English Physicist J.J. Thomson had shown that atoms contain some negatively charged particles of masses significantly smaller than that of the hydrogen atom. Nevertheless, the exact value of the charge carried by these subatomic particles remained in the dark. The very existence of these particles was not accepted by many due to a lack of concrete evidence. Thus, the atomic model was shrouded in mystery. In this scenario, with Millikan’s groundbreaking effort to quantify the charge on an electron, the atomic theory came of age in the early years of the twentieth century.
Controversy about the Oil Drop Experiment and Discovery
Robert Millikan was the sole recipient of the Nobel Prize in Physics in 1923 for both his work in this classic experiment and his research in the photoelectric effect . Fletcher’s work on the oil drop project, however, was not recognized. Many years later, the writings of Fletcher revealed that Millikan wished to take the sole credit for the discovery in exchange for granting him a Ph.D. and helping him secure a job after his graduation.
The beauty of the oil drop experiment lies in its simple and elegant demonstration of the quantization of charge along with measuring the elementary charge on an electron that finds widespread applications to this day. With the progress of time, considerable modifications have been made to the original setup resulting in obvious perfection in the results. Still, no substantial deviation from the results of the classical experiment could yet be found.
- Robert Millikan and Harvey Fletcher conducted the oil drop experiment to determine the charge of an electron. The experiment was the first direct and riveting measurement of the electric charge of a single electron.
- They suspended tiny charged droplets of oil between two metal electrodes by balancing downward gravitational force with upward drag and electric forces.
- They later used their findings to determine the mass of the electron.
- Kentchemistry.com
- Physics.utah.edu
- Nobelprize.org
- Ffden-2.phys.uaf.edu
- Chem.libretexts.org
Article was last reviewed on Thursday, February 2, 2023
Related articles

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
Oil Drop Experiment — Overview & Importance - Expii
Oil drop experiment — overview & importance, explanations (4), oil drop experiment.
- Designed by Robert Millikan at the University of Chicago
What was the experiment?
A fine mist of oil was sprayed above the top metal plate which contained a hole so that some of the oil droplets could pass through to the second metal plate
X rays were used to irradiate the droplets causing the electrons in the air to attach to the oil droplets (which caused the droplets to become charged)
The charge of an electron was found by observing the fall rate of the droplets and using that to calculate the charge of the electrons

Image source: By Caroline Monahan
Importance of this experiment
Determined the charge of an electron to be −1.60⋅10−19 C
Used the charge to calculate the mass of an electron to be 9.09⋅10−18 g (Calculated using Thompson's charge-to-mass ratio )
Related Lessons
Oil-Droplet Free Body Diagram

by T. Gibbons
Discovery of the Electron
Previously, we studied JJ Thomson's cathode ray tube experiment . He discovered the electron in 1897 . It was a massive scientific leap. Before Thomson, scientists presumed Dalton's atomic theory . Dalton was mostly correct. For example, elements have the same types of atoms . So, a chunk of pure gold consists of only gold atoms. He also realized that compounds are made of different atoms in whole-number ratios. But, he assumed that the atom was the smallest fundamental unit. With Thomson's discovery, we knew there were subatomic particles. Atoms had smaller building blocks! It would be many years before the discovery of the proton and neutron . But, he had opened up a new area of exploration in physical chemistry ! What are the properties of the electron?
Thomson took the first step. From the cathode ray tube experiment he calculated the electron's charge-to-mass ratio. He determined it was 1.78×108Cg. The units are coulombs per gram. The coulomb is the SI unit for a charge. But, a charge-to-mass ratio is only so helpful. What's the actual charge? What's the actual mass? There was still more work to do!
An Experiment to Find the Electron's Charge
Robert Millikan was the first scientist to develop an experiment to determine the electron's charge. He performed his famous oil drop experiment in 1909. The experimental setup was quite ingenious. He used a spray bottle to create aerosolized oil droplets. The oil got sprayed above a metal plate. The plate had a tiny hole. So, a few droplets passed through it. Below was a second plate. He connected the two plates to a voltage source. The voltage caused the upper plate to build a positive charge. The lower plate developed a negative charge. When the oil was between the plates, he would ionize them using x-ray radiation . The nitrogen , oxygen , and hydrogen , in the air, would lose electrons and transfer them to the oil. So, the droplets held negative charges.
First, he measured the velocity of the droplets without any voltage. At this point, he applied some knowledge from physics. At terminal velocity, the drag force equals the gravitational force. Both are related to the droplet's radius. That allowed him to calculate the volume . Then he used the oil's density to convert volume to mass. So, he knew the mass of the droplet.
Next, he turned on the voltage source. Remember, the droplets were ionized. So, they have an ionic charge . So, they experienced electrostatic interactions from the metal plates. He fine-tuned the voltage until the droplets became suspended in the air. That meant the gravitational force and the electric field were equal. Based on the electric field, he calculated the charge of the oil droplet. He compared droplets of various sizes. He observed that their charges were whole number integers of a specific value. He assumed that there could only be whole numbers of electrons. That's an excellent assumption because you can't have half an electron. So, he calculated a single electron's charge as −1.59×10−19C.
Calculating the Electron's Mass
Robert Millikan had determined the electron's charge! That's a huge accomplishment. With the charge, we can calculate the electron's mass. How? We can use the mass-to-charge ratio from the cathode ray tube experiment.
1g−1.78×108C×−1.59×10−19Ce−=8.93×10−28ge−
Modern Values
Are you wondering how close Millikan and Thomson were to our modern values? The current mass value is 9.10938×10−28g. That's really close to the result we just calculated! What about the charge? Our best result is 1.60217663×10−19. Millikan was within one percent ! That's not too bad for measuring oil drops! The difference from our modern value is caused by experimental error and uncertainty .

(Video) Robert Millikan's Oil Drop Experiment - The Charge of an Electron
by Ben's Chem Videos

In this video, Ben discusses how Robert Millikan found out the charge of one electron, as well as the mass of one single electron, through his oil drop experiment.

IMAGES
VIDEO
COMMENTS
Millikan oil-drop experiment, first direct and compelling measurement of the electric charge of a single electron.It was performed originally in 1909 by the American physicist Robert A. Millikan, who devised a straightforward method of measuring the minute electric charge that is present on many of the droplets in an oil mist. The force on any electric charge in an electric field is equal to ...
Millikan's setup for the oil drop experiment. The oil drop experiment was performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge (the charge of the electron). [1] [2] The experiment took place in the Ryerson Physical Laboratory at the University of Chicago.[3] [4] [5] Millikan received the Nobel Prize in Physics in 1923.
The Millikens Oil Drop Experiment was an experiment performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the charge of an electron. This experiment proved to be very crucial in the physics community. Millikens Oil Drop Experiment Definition
The experiment is conducted with this drop. Theory and Calculations. First, the oil drop is allowed to fall in the absence of an electric field, and its terminal velocity, say v 1, is found out. Using Stokes' law, the drag force acting on the drop is calculated using the following formula.
Balance of Forces: Newton's Law a : radius of drop ρ: density ρ= ρ oil -ρ air v: velocity of oil drop Q: charge of oil drop E: electric field E=V/d V : Voltage across plates η: viscosity of air g : gravitational const. Ö ()) 6 1) dr g dr a ag E g E Fa gz FQ dv F v t E d SK Ö zg 6SK vrag QEE Forces on the oil drop:
A description of the historic oil drop experiment that was performed by Robert Millikan to determine the electrical charge of the electron. Included is a diagram of the apparatus used and a discussion of the forces acting on the oil droplets.
The weight of the drop . In the initial observation the drop falls freely under gravity. If the drop has mass m the gravitational force acting on the drop is mg, where g is the acceleration due to gravity (the gravitational field). This force acts downwards. The downwards force is slightly altered because the effective mass of the drop m' is its actual mass m oil minus the mass of the air it ...
Oil drop experiment. V + - d 500V V g telescope atomizer Oil drops ∅~1m 𝝆𝒂 𝒓 Forces on the oil drop: 1) Gravity + buoyant force (air displaced by oil drop) 2) Drag force of the oil drop in the air 3) Electric force on oil drops which carry charge Q 2/11/2013 8
This experiment first described by [Millikan, 1913] is based on the fact that different forces act on an electrically charged oil drop moving in the homogeneous electric field of a plate capacitor (Figure 1). Measuring the effect of these forces on an oil droplet makes it possible to measure the effect of excess electric charge on the droplet.
An Experiment to Find the Electron's Charge. Robert Millikan was the first scientist to develop an experiment to determine the electron's charge. He performed his famous oil drop experiment in 1909. The experimental setup was quite ingenious. He used a spray bottle to create aerosolized oil droplets. The oil got sprayed above a metal plate. The ...