Cool Science Experiments Headquarters
Making Science Fun, Easy to Teach and Exciting to Learn!
Science Experiments

Floating Egg Science Experiment
Can you make an egg float in water? In this simple science experiment, we take just a few minutes to test the laws of density and discover just how easy it is to make an egg float!
Below you’ll find detailed instructions and our demonstration video as well as the scientific explanation of “why it works.” We’ve also included a more ideas to explore the concept a bit further.

JUMP TO SECTION: Instructions | Video Tutorial | How it Works
Supplies Needed
- 2 Tall Drinking Glass
Floating Egg Science Lab Kit – Only $5

Use our easy Floating Egg Science Lab Kit to grab your students’ attention without the stress of planning!
It’s everything you need to make science easy for teachers and fun for students — using inexpensive materials you probably already have in your storage closet!
Floating Egg Science Experiment Instructions
Experiment Setup – Start with some observations about the eggs. Note that they are both raw eggs and have a similar size and weight. Then ask some questions. Do you think that the eggs will sink or float when placed in water? Do you think it’s possible to make them float? If so, how? Write down your hypothesis (prediction) and then follow the steps below.

Step 1 – Fill a tall drinking glass about 3/4 full of water and carefully place the egg into the glass. What happens to the egg? That’s right, it sinks to the bottom.
Did you know there is a way to make it float? Continue on in the experiment to find out how.

Step 2 – Fill another tall drinking glass about 3/4 full of water.

Step 3 – Add 3 Tablespoons of salt to the water and stir until it is completely combined. What do you think will happen if you place the egg into the glass with the salt water? Write down your hypothesis (prediction) and then test it to see if you were right.

Step 5 – Next carefully place the second egg into the glass with the salt water. What happens to the egg? That’s right, it floats. Take a moment to make some observations. Why do you think one egg sinks and the other egg floats?
Find out the answer in the how does this experiment work section below.
Video Tutorial
How Does the Floating Egg Science Experiment Work
Why does the egg sink in regular tap water, but float in saltwater? The answer lies in the density of water!
Density is a measure of the mass per unit volume of a substance. Simply said, how much “stuff” in a given volume. Water has a density of 1 g/mL (g/cm3). Objects will float in water if their density is less than 1 g/mL. Objects will sink in water if their density is greater than 1 g/mL.
The egg will sink in regular tap water because the density of the egg is greater than the density of water. The egg’s density is only slightly higher than water at 1.03 g/mL, but that is enough to make the egg sink.
When you add salt to the water, you are increasing the density of the water by adding more mass (or stuff) in the given volume. You don’t really change the volume of the water by adding salt. By adding enough salt, you increase the density of the water so that it is higher than the density of the egg and the egg will float!
Other Ideas to Try
Try this experiment again, but instead of using an egg use a potato slice or a carrot slice. You will have to play around with the amount of salt you add to the water because all objects have their own unique density. Add salt a tablespoon at a time and mix well until you cannot see any salt in the solution, then add your object to see if it floats or sinks. Remove your object and keep adding salt until you can get your object to float. To make it a true science experiment, create a data table to keep track of how much salt you add to the solution.
I hope you enjoyed the experiment. Here are some printable instructions.

- Drinking Glass
Instructions
- Fill a tall drinking glass about 3/4 full of water
- Place the egg into the glass of watch and watch it sink
- Fill another tall drinking glass about 3/4 full of water
- Add 3 Tablespoons of Salt and stir until combined
- Place the egg into the glass and watch it float

Reader Interactions
April 3, 2019 at 2:58 pm
i love this experiment
January 23, 2020 at 11:14 pm
I really loved doing this experiment with my class
August 26, 2020 at 2:59 pm
The egg floats because the density of the salt water changes to be greater than the egg and the density of the egg becomes less dense so then the egg floats. But when you put an egg in tapwater the density of the egg is greater than the density of the tapwater which makes the egg sink.
January 20, 2022 at 11:33 am
bro I loved this experiment it was amazing!!! I tried it out with my friends and it worked! Thank you!
February 10, 2022 at 7:19 pm
this is very helpful thank you
March 7, 2022 at 9:56 am
i loved this experiment : )
April 16, 2023 at 11:35 am
I love doing this experiment at home
May 1, 2023 at 9:00 am
It’s amazing thank you for sharing.
November 3, 2023 at 10:18 am
This is my science fair experiment! YAY!
November 25, 2023 at 7:41 am
wow what a great experiment m!!!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- Privacy Policy
- Disclosure Policy
Copyright © 2024 · Cool Science Experiments HQ
April 10, 2014
Salty Science: Floating Eggs in Water
A density demonstration from Science Buddies
By Science Buddies
Key concepts Density Mass Volume Concentration Buoyancy Water Introduction Have you ever wondered why some objects float in water and others sink? It has to do with the density of the objects compared with the density of the water surrounding them. If an object is less dense than the water around it, it will float. Because salt water is denser than freshwater, some things float more easily in the ocean—or extremely salty bodies of the water, such as the Dead Sea. You can make your own dense water by adding salt to tap water. In fact, if you add enough salt, you can make the water so dense that an egg will actually float in it! Explore how this works in this science activity. Background If you put an egg in a cup of tap water, it will sink to the bottom. Why is this? Because the density of the egg is higher than the density of tap water, so it sinks. Density is the mass of a material per unit volume. For example, the density of freshwater under standard conditions is approximately one gram per cubic centimeter. But, if you add enough salt to the water, the egg will actually float back up to the surface! Adding salt to the water increases the density of the solution because the salt increases the mass without changing the volume very much. When enough salt is added to the water, the saltwater solution's density becomes higher than the egg's, so the egg will then float! The ability of something, like the egg, to float in water or some other liquid is known as buoyancy. But just how much salt is needed to make an egg float? In this science activity you'll figure that out by making solutions with varying concentrations of salt in them. Materials
Measuring cup
Large container, such as a large bowl or cooking pot (It must be able to hold at least three cups.)
One half cup of table salt
Five cups that hold at least 16 ounces each
Permanent marker (if you are using plastic cups) or masking tape and a pen (to label nondisposable cups)
Three spoons for mixing salty solutions
Soup spoon for egg transfers
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Preparation
Take the egg out of the refrigerator and allow it to warm to room temperature. Be sure to always wash your hands after handling uncooked eggs because they may carry salmonella.
Pour one and one half cups of water into your large container.
Add one half cup of salt to the large container and stir to dissolve some of the salt (it will not all dissolve yet).
Add one more cup of water to the large container (making two and one half cups total) and stir to dissolve the remaining salt. The salt should be completely dissolved before you go on to the next step. It may take several (five to 10) minutes of stirring, so you may need to be patient. Why do you think it's important to start out with a solution that has such a high concentration of salt?
Arrange the five cups on a surface, going in a line from left to right. Label the cups 1 to 5. If you are using plastic cups, you can use a permanent marker to label them. If you are using nondisposable cups, you can use masking tape and a pen to label them.
Add three quarters cup of the salty solution you prepared to cup 1.
Add three quarters cup of plain tap water to cups 2 through 5. (Cup 5 will be plain tap water.)
Add three quarters cup of the salty solution you prepared to cup 2 and mix it. What is the salt concentration in cup two compared with cup one?
Add three quarters cup of the salt solution from cup 2 to cup 3 and mix it. What is the salt concentration in cup 3 compared with cups 1 and 2?
Add three quarters cup of the salt solution from cup 3 to cup 4 and mix it. What is the salt concentration in cup 4 compared with the other cups?
Use a soup spoon to place an egg in cup 5. Does the egg float?
Use the spoon to take the egg out and place it in cup 4. Does the egg float?
Repeat this process with cups 3, 2 and then 1. In which cup does the egg first float? If the egg floated in more than one cup, did you notice any difference in how it floated? What does this tell you about the density of the egg?
Extra: In this science activity you figured out, within a factor of two, how much salt it takes to float an egg. You could narrow down the range further by testing additional saltwater solutions to try and determine the egg’s density. To do this, start your solution with the salt concentration in which the egg first floated and make a new dilution series, as you did before. Now in which cup does the egg first float? What does this tell you about the density of the egg?
Extra: Repeat this activity using several more eggs, possibly both hard-boiled and uncooked eggs. Do you get the same results with other eggs or is there some variation between different eggs? For testing hard-boiled versus raw eggs, you should test the same egg, first raw and then after hard-boiling it to investigate any differences.
Extra: Find out how much salt there is in seawater. From the results of your activity, do you think an egg would float or sink in seawater?
[break] Observations and results Did the egg float in cup 1 and 2, but not in cups 3, 4 or 5? You likely saw that the egg floated best in cup 1, floated a little less in cup 2 (but part of it was above the surface) and did not float in the other cups. Cup 1 had the undiluted salty solution that you originally prepared, which was one half cup of salt in two and one half cups water total. The concentrations of the salt solutions in cups 2 to 4 were halved as you increased in cup number; for example, the concentration of the salt in cup 2 was half that of cup 1, and the concentration of the salt in cup 3 was half again of cup 2. (Cup 5 had plain tap water.) The egg should have sunk in cups 3, 4 and 5 because the density of the egg was higher than the density of the solutions (or plain tap water) in those cups. Cups 1 and 2 had more salt in them than the other cups (with cup 1 having the most salt), which means these solutions were denser. The egg should have floated (with part of it above the water surface) in these two cups because the solutions were denser than the egg. The actual density of the egg is in between the density of the solution in cup 3 and that in cup 2. More to explore What Is Density? , from Charles E. Ophardt, Elmhurst College Why Is the Ocean Salty? , from Herbert Swenson, U.S. Geological Survey Publication Fun, Science Activities for You and Your Family , from Science Buddies How Salty Does the Sea Have to Be for an Egg to Float? , from Science Buddies
This activity brought to you in partnership with Science Buddies
Science Fun

Floating Egg

- Salt (1 – 2 cups)
- A tall drinking glass
Instructions:
1. Pour water into the glass until it is about half full. 2. Place an egg in the glass of water and see if it sinks or floats (it should sink). 2. Stir in lots of salt. Start with 1 tablespoon and stir it until the salt dissolves. Keep adding more salt until the egg floats. 3. Next, carefully pour more fresh water until the glass is nearly full (be careful to not disturb or mix the salty water with the plain water). If you’re very careful, you can get the egg to float between the fresh and saltwater!
VIDEO COMING SOON BUT YOU CAN STILL ENJOY THESE AWESOME EXPERIMENTS!
How It Works:
The egg is denser than the fresh water (more molecules per square inch), this causes it to sink. When you start dissolving salt in the water, this is increasing the density (adding more molecules per square inch). Eventually the water becomes denser than the egg causing the egg to float. When you carefully add fresh water again, this fresh water is less dense than the salt water so it floats right on top!
Extra Experiments:
Are there other liquids you can add to make the egg sink or float? What else can you dissolve in the water to make the egg float?
EXPLORE TONS OF FUN AND EASY SCIENCE EXPERIMENTS!

SUBSCRIBE AND NEVER MISS A NEW SCIENCE FUN VIDEO!
previous experiment
Next experiment.

Floating Egg Experiment
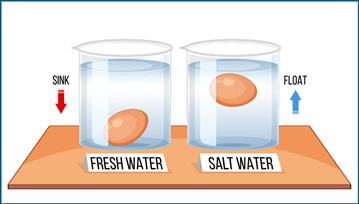
Will an egg float in fresh or salt water.

Will an egg float in fresh or salt water? The Floating Egg Experiment is an easy hands-on investigation that can be done in your own kitchen! I have used this lab before as a class demonstration, group lab investigation, and with my own kids at home. There are several science terms and concepts that can be taught in this experiment including density, solutions, mixtures, saturation, concentration, mass, and the list goes on. Scroll to the bottom to download your own Floating Egg Experiment Lab Sheet!
*Don’t miss out on the Salt Water Density Lab featuring a free observation lab sheet too! And be sure to check out all of our FREE Science Resources including our Labs & Experiments !
As a Christian Book and Amazon affiliate, we may earn commissions from qualifying purchases. Thank you for your support. As always, we only recommend items that we truly feel will benefit your homeschooling experience. We appreciate it.
Floating Egg Experiment Introduction
Before conducting the experiment, there are a few things you should cover with your students to create a meaningful learning experience. First, spend time talking about the terms below. We discuss the meaning of salinity and a homogeneous mixture amongst other concepts. Since this investigation is demonstrating the difference in density between salt water (oceans) and fresh water (rivers, lakes, ice), it works well with our Water Distribution Unit .

Important Terms and Facts
– Salinity describes the amount of salt dissolved in water. Saline water is water with salt dissolved in it.
– A homogeneous mixture is a type of mixture where a solute (salt) is dissolved into a solvent (water). This type of homogeneous mixture is called a solution .
– Density is the amount of mass in volume. Volume is the amount of space something takes up.
– Buoyancy is a force on an object making that object rise or move forward.
Floating Egg Experiment Lab Sheets
Write hypothesis.

Once you have introduced the key terms in the Floating Egg Experiment, make sure the students are given the following facts and the FREE FLOATING EGG EXPERIMENT LAB SHEETS.
- Water has a density of 1 g/mL (g/cm3).
- Objects will float in water if their density is less than 1 g/mL.
- Lastly, objects will sink in water if their density is greater than 1 g/mL.
After giving your students this information, guide them to write a hypothesis for the posing question, “Will an egg float in salt or fresh water?”
For example, their hypothesis could read like this:
If I add 1 TBSP of salt to every ½ cup of fresh water, a raw egg _____________________________ float.
Students will plug in the words, “will” or “will not,” in the blank of the hypothesis.
Identify Variables

Identifying variables in an experiment is something that takes most students practice. It is a good idea to continuously review the following terms. Check out our Scientific Method unit to get more practice.
Manipulated Variable – variable that is different in the experiment. It is the thing that is manipulated. *In this case, the manipulated variable is the amount of salt added to the water.
Responding Variable – variable that is being tested. It responds to the manipulated variable. *Hence, if the egg floats or not.
Controlled Variable – variable(s) in the experiment that do not change or remain the same. *In this lab, the glasses and raw egg are the controlled variables.
Review the experiment before conducting...
For you teachers, it is always advisable to try an experiment out first to ensure that it works the way it is supposed to. There is nothing worse than demonstrating in front of a group of students for an experiment to flop! It has happened to the best of us… yes, it has happened to me! Watch this video I found on YouTube to get a step by step of the experiment. It is pretty easy to demonstrate.
I have used this experiment for years with the exception of the salt water with the fresh water on top. That aspect of the experiment was new to me. So, I too tried this at home before showing it to my students. *I wonder if it will work with glasses that aren’t quite so narrow at the bottom? Let’s see…
Gather Materials

The materials list is likely to all be found within your kitchen. I love experiments that do not require a tricky supply list. So, gather 3 raw eggs, water, salt, a spoon, 3 clear glasses, and a measuring cup and tablespoon come in handy.
Fresh Water first and then Salt Water

I always start demonstrating the egg sinking in the fresh water first. Next, you will show how adding salt to the fresh water will change its density. Thus, the egg will now float in the denser salt water. In the Egg Floating Experiment, I use 1 tablespoon of salt per 1/2 cup of water. This enables the students to measure more precisely. Some kids need the restrictions.
*On their lab sheets, students will draw and record observations and make conclusions.
Extended Learning...

This aspect of the Floating Egg Experiment is a new for me. I never did this in years past, but what a great addition. You can choose to demonstrate this last glass or the students can continue to follow the procedures on their lab sheet. In the end, take the time to talk about the science behind this experiment. This is where the science comes all together!
What's the Science behind it?
It’s very important that before, during, and after an experiment that you are explaining the science behind the experiment. If you skip this step, then the lab becomes merely a fun hands-on activity with no real science connections. The experiments are meant to help the students get past the surface and begin to soak in the abstract and unseen science behind it.
Glass #1 – The egg sunk to the bottom in glass #1 because the egg is more dense than the water. Hence, the egg has more mass in its volume than the water does.
Glass #2 – The egg floats in salt water because the salt water is more dense than the egg. This means that the salt water has more mass in its volume than the egg does. The salt adds mass to the volume of the water creating a more dense liquid.
Glass #3 – When the egg is put in the cup, the glass is only half full with a mixture of salt and water. This causes the egg to float in the salt water instead of sinking. When plain water is added to the salt water, the egg remains in the middle of the glass because of density. The plain water is the least dense, then the egg, and the most dense is the salt water at the bottom of the glass.
*When the floating eggs are pushed into the salt water, the eggs float right back up to the top of the salt water. This is due to buoyancy. The force of the salt water causes the egg to rise back up.
DOWNLOAD FLOATING EGG EXPERIMENT LAB SHEETS
Check out these other free resources.

John 4:14 – But whosoever drinketh of the water that I shall give him shall never thirst; but the water that I shall give him shall be in him a well of water springing up into everlasting life.

Share this:

Reading & Math for K-5
- Kindergarten
- Learning numbers
- Comparing numbers
- Place Value
- Roman numerals
- Subtraction
- Multiplication
- Order of operations
- Drills & practice
- Measurement
- Factoring & prime factors
- Proportions
- Shape & geometry
- Data & graphing
- Word problems
- Children's stories
- Leveled stories
- Sight words
- Sentences & passages
- Context clues
- Cause & effect
- Compare & contrast
- Fact vs. fiction
- Fact vs. opinion
- Main idea & details
- Story elements
- Conclusions & inferences
- Sounds & phonics
- Words & vocabulary
- Reading comprehension
- Early writing
- Numbers & counting
- Simple math
- Social skills
- Other activities
- Dolch sight words
- Fry sight words
- Multiple meaning words
- Prefixes & suffixes
- Vocabulary cards
- Other parts of speech
- Punctuation
- Capitalization
- Narrative writing
- Opinion writing
- Informative writing
- Cursive alphabet
- Cursive letters
- Cursive letter joins
- Cursive words
- Cursive sentences
- Cursive passages
- Grammar & Writing
Breadcrumbs
Floating Egg Science Experiment
Here are the steps to perform the experiment:
Materials needed:
A glass or container
Instructions
Fill the glass or container with water, leaving some space at the top.
Gently place the egg in the water. The egg should sink to the bottom.
Take the egg out of the water.
Start adding salt to the water, a tablespoon at a time, and stir until the salt dissolves.
Place the egg back in the water after each addition of salt and observe what happens.
Keep adding salt until the egg floats to the surface and remains there.
Explanation
The density of water increases as salt is added to it, making the water denser (more molecules per square inch). When the egg is placed in the plain water, it sinks to the bottom because the density of the egg is greater than that of the water. However, as salt is added, the water becomes denser than the egg, causing the egg to float to the surface.
This experiment illustrates the concept of buoyancy and the Archimedes' principle, which states that the buoyant force on an object is equal to the weight of the water displaced by the object. The egg floats because it displaces a volume of water that weighs more than the egg.
This content is available to members only.
Join K5 to save time, skip ads and access more content. Learn More

Floating Egg Science Experiment ( Using Salt, Sugar & Saline Water)
- December 10, 2020
- 10 Minute Science , 5-6 Year Olds , 7-9 Year Olds , Physics , Rainy Day Science
Hello everyone! Today we have come up with simple ‘ Floating Egg Science Experiment with a twist’ that can be done within 5-10 minutes.
My kids call it as a pre-breakfast activity. This is an easy and funny experiment to teach density for kids .

We know that some things float in the water and some others not. Do u know why the things sink in the water!? Let us learn something about floating science using eggs.
Floating Egg Experiment
This activity is a cool way to learn the concept – density! We are going to perform the experiment with four different liquids to understand the science behind floating objects in water.
We commonly see that eggs sink when we put in the water. What is the reason behind this!? Does egg sinks the same way when dropped in other liquids? We will perform a simple activity to learn the science behind it.
Try our 20+ Egg Science Experiments
Materials Required for the Activity

- Saline water (You can find saline water in any of the local pharmacies)
- Four glass jars (Either you take glass jars or beakers, make sure they are tall and wide enough to drop an egg)
- Four Raw Eggs (Ensure the eggs are not broken or given any crack to avoid the unnecessary mess with the leaky eggs during experiment)
- Fill one tall drinking glass or glass beaker about ¾ full of water.

- In the same way, fill the other glass with salt water. To prepare salt water, put 1-2 cups of salt in 500ml of water. Stir it with the spoon. That’s it. You are done with making salt water.

- Now it is second drinking glass turn! Fill it with sugar water. Prepare the sugar water same like how we made salt water in previous step.

- Saline water! Yes, we are using saline water as well to observe the floating science with eggs. Fill the fourth glass beaker with saline water.

- Finally, we have arrived to the kid’s favourite step i.e. dropping egg into the tall drinking glasses.

My younger daughter is eagerly waiting for my instructions to drop the egg in the liquids. When I said so, she carefully dropped the raw eggs into the four glasses filled with four different liquids each.

Ask your kids to observe the results that in which liquid the dropped eggs are floating or sinking.
On the initial test, we only had egg floating in the salt water. The sugar water was not dense enough to make the egg float. So we tried to add more sugar to the already prepared sugar solution.

Finally we made the egg float in the sugar water as the water is now more dense due to the added sugar.

How Does the Floating Egg Science Experiment Work?
Let us discuss the results of our experiment. The raw egg dropped in the tap water sinks immediately as soon as it is dropped. On the other hand the egg in the salt water floats.
We observe the same results with the sugar water as well. The raw egg floats nicely in sugar water as well.
How about our egg in the saline water? The egg didn’t float surprisingly in the saline water.
Now let us discuss on what made the eggs in salt & sugar water float and why the egg in normal tap water and saline water sink!?

Science behind floating egg
The egg in the glass of regular tap water sinks to the bottom because the density of egg is more compared to density of water.
Why the egg in salt water floats? When the salt is added to the water, it increases the density of the water and hence the density of the egg slowly becomes lesser than the salt water.
You are dissolving the more the salt into the water means you are increasing the density of water. The denser the liquid is the easier for the object in the water to float.
The same formula applies to the sugar water. The density of sugar water is more than the density of egg.
On the other hand, saline water is made of salt and water. However, the density of the saline water that we used seems to be lower than than egg. Thus it floated in the sugar water and sinked in the saline water.
Density is a concept dealing with how closely a substance is packed to be together.
We will compare this concept with our daily life things. For example: consider we have two bowls one is filled with salad and the other is with rice.
Both are of taken in same quantity but we feel the salad bowl is lighter than the rice bowl because the ingredients are packed tightly in the rice bowl than the salad of lettuce and vegetables which are very light in nature.
In the same way, the molecules in the salt, sugar, and saline water are packed more closely and makes the salt, sugar, and saline water denser than the water where the molecules are packed lighter thus making it less denser.
Even the egg has some density but less than the salt water and hence the egg floats in salt, sugar, and saline water.
So, when you go to swimming pool or beach or ocean, observe that you will float easily and lightly compared to salt water. The denser the liquid, the easier you will float! Amazing right!?
Experiment Extensions
Try different liquids and different substances to dissolve in the water in order to make an egg float.
Try these Density Science Experiments :
9 Layer Density Tower
Hot & Cold Water Experiment
DIY Sugar Density Rainbow
Oil, Food Color & Water – Fireworks
How to make Lava Lamp
Subscribe to our newsletter to be the first one to know our science experiments as soon as we publish them.
Leave a Reply Cancel Reply
Your email address will not be published. Required fields are marked *
Name *
Email *
Add Comment *
Save my name, email, and website in this browser for the next time I comment.
Post Comment

IMAGES
COMMENTS
In this simple science experiment, we take just a few minutes to test the laws of density and discover just how easy it is to make an egg float! Below you’ll find detailed instructions and our demonstration video as well as the scientific explanation of “why it works.”
When enough salt is added to the water, the saltwater solution’s density is higher than the egg’s, and the egg will then float! The ability of something, like the egg, to float in water or some other liquid is known as buoyancy. But just how much salt is needed to make an egg float?
Apr 10, 2014 · In fact, if you add enough salt, you can make the water so dense that an egg will actually float in it! Explore how this works in this science activity. If you put an egg in a cup of tap...
Floating Egg. What happens when you put an egg in a glass of regular water? This is a cool way to learn about density. Materials: One egg; Water; Salt (1 – 2 cups) A tall drinking glass; A spoon . Instructions: 1. Pour water into the glass until it is about half full. 2. Place an egg in the glass of water and see if it sinks or floats (it ...
Will an egg float in fresh or salt water? The Floating Egg Experiment is an easy hands-on investigation that can be done in your own kitchen! I have used this lab before as a class demonstration, group lab investigation, and with my own kids at home.
• Develop a hypothesis for which container (salt or fresh water) will cause the egg to float. • Fill the two containers with equal amounts of tap water. • Add a raw egg to each container. • Record if the egg floats in a data table. • Add 1 tablespoons of table salt at a time to the “salt water” container and stir until salt is dissolved.
Mar 2, 2023 · This is a classic science experiment that shows how changes in the density of a liquid can affect whether an egg sinks or floats. When you place an egg in regular water, it will sink to the bottom of the glass because the egg is denser than the water.
This experiment illustrates the concept of buoyancy and the Archimedes' principle, which states that the buoyant force on an object is equal to the weight of the water displaced by the object. The egg floats because it displaces a volume of water that weighs more than the egg.
Dec 10, 2020 · ‘Floating Egg Science Experiment’ with a twist - Try out with Salt water, sugar water, tap water and saline water. Add a little science to your kids morning breakfast before the egg becomes a delicious scrambled or omelette.
In this experiment, you'll use eggs (and a few scoops of salt) to study the science of floating. Will an egg float better in salt water or fresh water? To begin your floating eggs experiment, fill your two containers with water. Make sure the amounts are equal.