- Research article
- Open access
- Published: 30 November 2020

Machine learning approaches classify clinical malaria outcomes based on haematological parameters
- Collins M. Morang’a 1 ,
- Lucas Amenga–Etego 1 ,
- Saikou Y. Bah 1 , 2 ,
- Vincent Appiah 1 ,
- Dominic S. Y. Amuzu 1 ,
- Nicholas Amoako 1 ,
- James Abugri 3 ,
- Abraham R. Oduro 4 ,
- Aubrey J. Cunnington 5 ,
- Gordon A. Awandare 1 &
- Thomas D. Otto ORCID: orcid.org/0000-0002-1246-7404 6
BMC Medicine volume 18 , Article number: 375 ( 2020 ) Cite this article
10k Accesses
19 Citations
14 Altmetric
Metrics details
Malaria is still a major global health burden, with more than 3.2 billion people in 91 countries remaining at risk of the disease. Accurately distinguishing malaria from other diseases, especially uncomplicated malaria (UM) from non-malarial infections (nMI), remains a challenge. Furthermore, the success of rapid diagnostic tests (RDTs) is threatened by Pfhrp2/3 deletions and decreased sensitivity at low parasitaemia. Analysis of haematological indices can be used to support the identification of possible malaria cases for further diagnosis, especially in travellers returning from endemic areas. As a new application for precision medicine, we aimed to evaluate machine learning (ML) approaches that can accurately classify nMI, UM, and severe malaria (SM) using haematological parameters.
We obtained haematological data from 2,207 participants collected in Ghana: nMI ( n = 978), SM ( n = 526), and UM ( n = 703). Six different ML approaches were tested, to select the best approach. An artificial neural network (ANN) with three hidden layers was used for multi-classification of UM, SM, and uMI. Binary classifiers were developed to further identify the parameters that can distinguish UM or SM from nMI. Local interpretable model-agnostic explanations (LIME) were used to explain the binary classifiers.
The multi-classification model had greater than 85% training and testing accuracy to distinguish clinical malaria from nMI. To distinguish UM from nMI, our approach identified platelet counts, red blood cell (RBC) counts, lymphocyte counts, and percentages as the top classifiers of UM with 0.801 test accuracy (AUC = 0.866 and F1 score = 0.747). To distinguish SM from nMI, the classifier had a test accuracy of 0.96 (AUC = 0.983 and F1 score = 0.944) with mean platelet volume and mean cell volume being the unique classifiers of SM. Random forest was used to confirm the classifications, and it showed that platelet and RBC counts were the major classifiers of UM, regardless of possible confounders such as patient age and sampling location.
The study provides proof of concept methods that classify UM and SM from nMI, showing that the ML approach is a feasible tool for clinical decision support. In the future, ML approaches could be incorporated into clinical decision-support algorithms for the diagnosis of acute febrile illness and monitoring response to acute SM treatment particularly in endemic settings.
Peer Review reports
In 2018, there were 228 million cases of malaria worldwide, 93% of which occurred in the African region [ 1 ]. Furthermore, approximately 450,000 deaths were reported, of which 61% were children under 5 years old [ 1 ]. According to WHO 2018 report, over 2.7 billion US dollars were spent towards various control and elimination efforts to address the global burden of malaria [ 1 ]. This includes over 2.74 billion doses of artemisinin-based combination therapies, procured in 2017 [ 1 ]. Unfortunately, incorrect diagnosis leads to incorrect treatment. It can increase the chances of antimalarial drug resistance, or for false negative diagnosis, it may result in misdiagnosis of malaria, inappropriate treatment, and progress to severe disease or death [ 2 , 3 , 4 ]. The gold standard for malaria diagnosis is microscopy, which requires extensive training, but rapid diagnostic tests (RDTs) have become the frontline diagnostic tools for malaria because of their ease of use at point-of-care [ 5 ].
One drawback of RDTs is the emergence of gene deletions of the target antigen, histidine-rich protein ( Pfhrp2/3 ) in the parasite genome, which render parasites undetectable by the most common RDTs [ 6 ]. Other challenges include insufficient sensitivity to detect low-level parasitaemia and the number of tests which need to be performed per positive result in settings with declining or low transmission [ 2 , 6 , 7 ]. Different problems are faced in non-endemic countries, where imported malaria must be suspected as a possible cause of fever before an RDT or microscopy would be performed in the first place, and failure to identify cases at first contact with health services often results in worse clinical outcomes [ 8 , 9 ]. Therefore, improved and complementary malaria diagnostic techniques are required, which can overcome some or all of these limitations.
Complete blood counts (CBCs) are the most commonly performed laboratory test in most hospitals in both developing and developed countries. The CBC is usually relied upon to provide clues for the diagnosis of patients where advanced methods for detection of specific diseases are lacking, with a parameter such as decreased platelet counts often associated with severe malaria (SM) [ 10 , 11 ]. In addition, haemoglobin (Hb) levels are very important for the classification of SM cases [ 12 ]. Indeed, the changes in haematological parameters during clinical malaria have been studied extensively to aid in the understanding of disease pathogenesis [ 13 , 14 , 15 , 16 , 17 , 18 , 19 ]. However, the potential and diagnostic value of haematological parameters measured by commonly available automated haematology analysers has not been fully studied using unbiased approaches such as machine learning (ML) techniques. These haematological parameters have the potential to be used in differentiating clinical malaria from other febrile illnesses, especially in areas where the reliability of RDTs is challenged by the high prevalence of Pfhrp2/3 deletion mutant parasites.
ML approaches use algorithms based on statistical assumptions and mathematical rules to learn patterns and produce meaningful classifications based on the association of each variable with the disease outcome [ 20 , 21 , 22 , 23 , 24 ]. These classifications can then be applied to new disease cases to make classifications on the most probable cause. This classification capability of ML has not been extensively implemented in the diagnosis of clinical malaria. To date, only a single study has reported the use of ML to diagnose malaria using clinical history and symptoms captured verbally and visually [ 25 ]. The sample size ( n = 376) was very small to deduce meaningful classifications, and the author concluded that more work would be needed [ 25 ]. Despite this, there have been far reaching studies on the application of ML in other areas of malaria research [ 26 , 27 , 28 , 29 , 30 ]. The diagnosis of malaria using ML on clinical datasets has been impaired by the lack of large data, as well as difficulty in data curation. Moreover, classical modelling is prone to over-fitting or under-fitting of data [ 31 ], but recent approaches such as imputation, encoding, centering and scaling of variables, and model optimization [ 24 ] enable augmented use of ML in malaria classification.
We hypothesized that we can classify clinical malaria and non-malarial infections (nMI) with an ML approach. We first collected and curated data from 2,207 patients including nMI ( n = 978), uncomplicated malaria (UM) ( n = 703), and SM ( n = 526). We generated ML models to classify clinical malaria (UM and SM) from nMI using haematological parameters.
Study population and sample collection
Standards for Reporting Diagnostic Accuracy Studies (STARD) guidelines [ 32 , 33 ] were followed in this study. The current study utilizes unpublished data of 526 patients from a previous case-control study of SM conducted by the Navrongo Health Research Centre (NHRC) located in the Kassena-Nankana Districts (KNDs) in the Upper East Region of Northern Ghana. In the original study, children with acute febrile symptoms admitted to the Navrongo War Memorial Hospital (NWMH), the only referral facility in the KNDs, were evaluated for inclusion into the study from August to December 2002 and May 2003 to April 2004. Full details of the study procedure, inclusion criteria, and demographic and clinical characteristics of SM cases may be reviewed in Oduro et al. [ 34 ].
In brief, the inclusion criteria for SM cases were (1) all children between 6 and 59 months who had fever (or history of fever in the past 24 h) and were admitted to the NWMH, (2) residence in the Navrongo Health and Demographic Surveillance System area [ 34 ], and (3) willingness of parents/caregivers to offer informed consent. Criteria for SM diagnosis and enrollment into the original study were classified as having SM by the WHO standard guidelines that include haemoglobin < 5 g/dL or haematocrit < 15% [ 34 , 35 ]. Ethical approval for the SM study was obtained from the NHRC Institutional Review Board (IRB), Noguchi Memorial Institute of Medical Research (NMIMR) IRB, Naval Medical Research Center IRB, and Ghana Health Service (GHS) Ethics Review Committee (ERC). Informed consent was obtained and documented, followed by administration of a questionnaire about the presenting symptoms and clinical examinations. Participants who did not consent and meet the study inclusion criteria and those who had reported taking antimalarial treatment in the past 2 weeks were excluded from the study, while those who turned out to be malaria negative by standard microscopy were withdrawn from the study. All study samples were taken prior to initiation of treatment except for samples taken for clinical monitoring during admission or for follow-up after discharge from the hospital.
The nMI and UM participants were recruited in a hospital-based cross-sectional study involving two hospitals: Kintampo North-Municipal Hospital, Kintampo, and Ledzokuku Krowor Municipal Assembly Hospital (LEKMA), Accra. The inclusion criteria were (1) outpatient children 1–15 years old, (2) presenting with fever or history of fever in the past 24 h or axillary temperature ≥ 38 °C, (3) and (4) signed informed consent by self (adolescents) and parent/guardian. The exclusion criterion was participants with known chronic disease or history of antimalarial drug use in the past 2 weeks. Ethical approval was also obtained from NMIMR-IRB, GHS-ERC, and Kintampo Health Research Centre (KHRC) ERC. A case was defined as nMI if the individual presenting to the hospital was malaria negative by either RDTs, Taqman array, or microscopy. Clinical data such as age, sex, and body temperature and symptoms such as fever were collected on recruitment.
Sample collection procedure
Venous blood was collected in the ante-cubital fossa. Tourniquet was not applied beyond 1 min during venesection to avoid haemo-concentration, which could give erroneous results for all parameters measured. Samples were taken mostly between 8 am and 12 pm to avoid variations due to individuals’ activity (such as rehydration and food intake). Samples (5 mL) were taken into K3 EDTA tubes (BD Vacutainer; Becton Dickinson, NJ, USA). Samples that could not get analysed within 2 h from the time of collection were stabilized at 2–8 °C to avoid changes that could occur in some haematological parameters should the sample be left on the bench for more than 3 h. Samples were analysed not later than 24 h from the time of sample storage at 2–8 °C. No capillary blood sample was taken during the study as it presents with subtle variations from venous blood parameters. CBC analysis was performed using the automated ABX Micros 60 haematology analyser, which measures white blood cell parameters, red blood parameters, and platelet parameters (Additional file 1 : Table S1). Data were manually cross-referenced twice for accuracy to ensure consistency in sample collection procedures.
Statistical classifier: median split
Kernel density estimation, which is a non-parametric technique, was used to estimate the probability density function of each haematological parameter and kernel distribution for each parameter between nMI, UM, and SM and visualized using density plots in R (R version 4.0.2). The median value within each diagnostic group (nMI, UM, and SM) was computed, and the mean of any two group medians was used for ‘median split’ to generate a dichotomous variable for each parameter (low and high levels representing below and above median, respectively) [ 36 ]. Contingency tables were used to summarize the relationship between clinical diagnosis (nMI, UM, and SM), and each dichotomous parameter. The generalized linear models for predictive analysis were used to explain the relationship between the clinical diagnosis and the dichotomous parameter. Odds ratios were computed through the exponent of the regression coefficients (logits) to estimate the strength of the relationship. Any OR with 95% confidence interval (CI) that includes a null value (1.0) indicated that the parameter was not significantly associated with clinical diagnosis. ANOVA was used to compare the model with the null model and chi-square test used to compute the degree of significance. All the analyses were done in R-software (R version 4.0.2).
Data pre-processing and normalization
A multivariate imputation via chained equations (MICE) plot was used to visualize the missing observations in the data. It was difficult to determine whether the missing values were missing ‘completely at random’ or ‘missing at random’ or ‘not at random’ to enable selection of the imputation method. Therefore, the demographic/clinical data and microscopy results were not imputed and were not used for modelling. The majority of the haematological parameters had less than 5% missing data, and the missing values were imputed using MICE package in R. Each variable in the training and test data was transformed using the Yeo-Johnson function, centred to have a mean of 0, and scaled to have a standard deviation of 1. The original dataset (before pre-processing and normalization) is available in Additional file 2 : Table S2.
- Machine learning
Six ML algorithms were evaluated to identify the best algorithm that can classify the binary data. These include partial least squares (PLS) logistic regression, multiple adaptive regression splines (MARS), random forest, decision trees, support vector machine, and artificial neural networks (ANN). PLS logistic regression was implemented by reducing the dimension of haematological parameters so as to increase accuracy. We used 10-fold cross-validation while tuning through 16 principal components (PC), whereby the optimal model used 2 PC. The optimal hyperparameters for MARS (with cross-validation) were determined in a grid search of 30 different combinations of 3rd degree and sampling 1000 terms to retain the final model [ 37 ]. Decision tree was implemented with the rpart function, which performs auto-tuning with an optimal subtree of 10 total tree splits. Random forest and support vector machines were implemented by first performing a grid search to identify the optimal hyperparameters followed by classification analysis. Three ANN were developed, one multi-classification ANN (nMI vs UM vs SM) and two binary classifications denoted as ANN (UM and nMI) and ANN (SM and nMI). For each ML model, the data were split into 80% training and 20% testing. The outcome was the clinical diagnosis of the participant (as concluded by the attending clinicians) having either UM or nMI or SM. Haemoglobin and haematocrit levels were not included in the modelling because they are used to support the diagnosis of malaria [ 10 , 19 , 35 , 38 ].
Hyperparameter tuning for artificial neural networks
The ANN was composed of an input layer of 15 haematological parameters. The loss was computed using categorical cross-entropy for the multi-classifier and binary cross-entropy for binary classifiers, while accuracy was used as the main evaluation metric. During training, the 80% training data was further split into 70% training and 30% validation with randomization (Fig. 1 ). Tensor board visualizations were used to check the dynamic graphs of our training and test metrics. Hyperparameters were tuned to identify the optimal model parameters for each classification. A hyper-grid was developed that adjusts the model capacity, normalization term, kernel regularization, and learning rate. To maximize the validation error performance, we tuned 12, 32, 64, 128, 256, and 512 rectified linear units (ReLU) in three hidden layers. We used batch normalization on each hidden layer for gradient propagation and performance improvement. We varied the dropout rate from 0.1, 0.2, 0.3, and 0.4 in all the three layers to identify the best dropout regularization that prevents the model from latching to happenstance patterns that are not significant. We used ‘Adam’ as the optimizer, but we varied the learning rate (0.1, 0.05, 0.001, and 0.0001) to find a global minimum. The tfruns R package was used to implement the hyper-grid in R-software, using 500 epochs, batch size of 64, and validation split of 0.3. These Keras models were initialized for all the three classifications to select the optimal model.
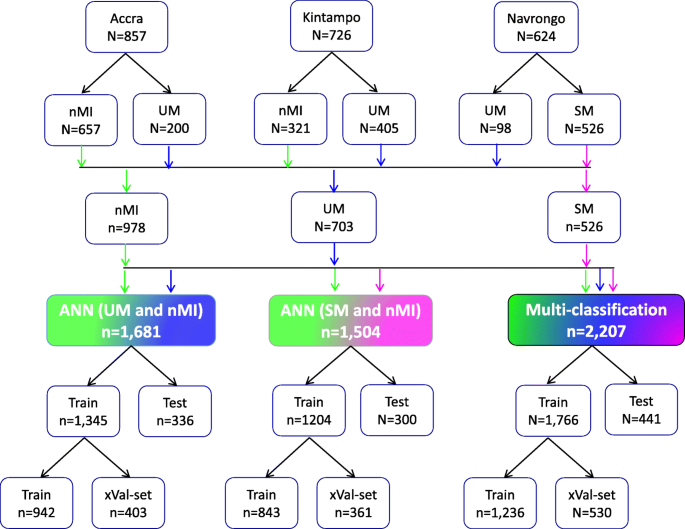
Study population and data splitting for building the ANN for clinical malaria. Samples were collected from one low transmission area (Accra, n = 857) and two high transmission areas: Kintampo ( n = 726) and Navrongo ( n = 624). The nMI ( n = 978) were collected from Kintampo and Accra and UM ( n = 703) were collected from all three areas, while the SM ( n = 526) samples were collected from Navrongo. A multi-classification ANN model was developed for nMI, UM, and SM, which was further evaluated by binary ANN models (1) ANN (UM vs nMI) and (2) ANN (SM vs nMI). For each model, data splitting was achieved by dividing data in an 80:20% ratio into training (Train) and testing (Test). The 80% training data was further split into a 70:30% ratio for training (Train) and cross-validation (xVal-set)
Model evaluations
Yardstick package was used to perform classifications on the test data as well as compute the performance of the model. The confusion matrix, accuracy, area under the receiver operating characteristic curve (AUC), precision and recall, and F1 score were the metrics used to evaluate performance. The F1 score is a measure of test data accuracy, which is a weighted average between precision and recall. To explain the model, we used local interpretable model-agnostic explanations (LIME Package in R) [ 39 ]. The classification model was set up, and an ‘explainer’ of the classifying model was initiated using the training data and the model output classifications. The explainer was used to explain the results of the test dataset as classification explanations (feature weights). The feature weights were used to build a heatmap for each ANN indicating how each feature explains the model.
Effect of patient age and sampling location on the model predictions
To test if patient age and sampling location significantly affected the models, we used three models: (1) a model for all the UM and nMI cases ( n = 1681), (2) a model for UM and nMI from Kintampo cases only ( n = 726), and (3) a model for only Kintampo cases and ages under 4 years ( n = 416). We tested the possibility of using the ANN to evaluate the models but there was some level of over-fitting and under-fitting of the 2nd and 3rd models, due to sample size limitation. Therefore, random forest was subsequently used, because of (1) its robustness to smaller sample size with minimal over-fitting of the data and (2) its ability to reduce the high variance from decision trees by combining several trees into one ensemble tree [ 40 ].
Statistical analysis
The clinical categorical data was analysed using Pearson’s chi-square while the continuous data such as the haematological parameters were analysed using the Kruskal-Wallis test with Dunn’s post hoc tests across the three groups (UM, SM, and nMI). All tests were two sided, and statistical significance was set at P < 0.05 for all analyses with adjustment for multiple testing. Data analyses were performed using R-software (R version 4.0.2), R-studio (version 1.1), and Python (version 2.7). The R codes with the methods, including the curated data files, can be found on github: https://github.com/misita-falcon/Machine-Learning-in-Clinical-Malaria .
Characteristics of the study participants
Participants were recruited as follows: 38.8% (857/2,207) from Accra, 32.9% (726/2,207) from Kintampo, and 28.3% (624/2,207) from Navrongo (Fig. 1 ). These participants from all the three locations constitute 44.3% (978/2,207) nMI, 31.8% (703/2,207) for UM, and 23.8% (526/2,207) for SM cases (Fig. 1 ). The median age was 3 years (range 2–6 years) for nMI, 4 years (range 2–7 years) for UM, and 1 year (range 1–2 years) for SM. The median ages were significantly different as determined by the Kruskal-Wallis test ( P < 0.001) (Table 1 ). The SM cases had a significantly higher median body temperature (38.3; range = 37.5–39.2), compared to the nMI (37.2; range = 36.5–38.4) and UM (38.1; range = 37–39) ( P < 0.001). There was a significant difference in proportions of individuals ( P < 0.001) among nMI, UM, and SM from different locations (Kintampo, Navrongo, and Accra) as determined by the chi-square analysis (Table 1 ). There was no association between sex and clinical diagnosis, although the number of females was higher than males in all three groups ( P = 0.247); nMI was 51.2% (501/978), UM was 54.9% (386/703), and SM was 55.1% (290/526) (Table 1 ). Fever was more common in SM (99.2%, 522/526) compared to UM (85.5%, 601/703) and lowest in nMI (59.4%, 581/978), and the chi-square analysis shows that there was an association between fever and clinical diagnosis ( P < 0.001) (Table 1 ).
Participants with UM had a higher geometric mean parasite density (27,467.59 parasites/μL, SD = 8.44) compared to SM individuals (16,674.41 parasites/μL, SD = 8.61). But, the median levels did not vary significantly between the two groups ( P = 0.592) (Table 1 ). Participants with nMI were negative by microscopy, RDT, and Taqman array. There were 212 different suspected infections in the nMI group, and the top 10 include upper respiratory tract infections (17%, 167/978), malaria (9.5%, 93/978), gastroenteritis (7.6%, 75/978), sepsis (6.1%, 60/978), otitis media (5.9%, 58/978), enteric fever (2.6%, 26/978), fever (2.1%, 23/978), tonsillitis (2.3%, 23/978), pneumonia (2.1%, 21/978), and anaemia (1.9%, 19/978) (Additional file 1 : Fig. S1). Laboratory results indicated that majority of the samples were undetermined/not available/not known (96%, 937/978), with only 4% having accurate laboratory results (41/978). Some of the organisms that were laboratory confirmed include Streptococcus pneumonia , Staphylococcus aureus , Salmonella typhi , Coxiella burnetii , and dengue virus (Fig. 2 ). Only 2 UM participants had co-infections (laboratory confirmed) with P. falciparum , and these individuals had Salmonella typhi and group D streptococcus . Since the sample size of laboratory-confirmed nMI cases was low, all the samples were grouped as nMI, instead of individual diseases during ML classifications.
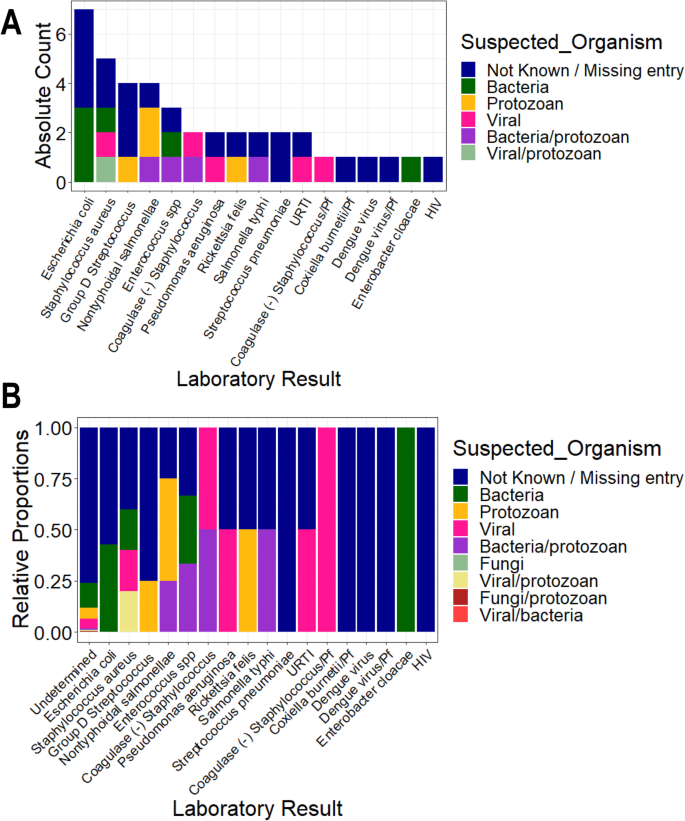
Clinical manifestations using laboratory diagnosis compared to various suspected infections by clinicians. Blood, urine, and stool samples were collected from majority of the individuals who were categorized as nMI. Cultures of either blood, urine, or stool were performed, depending on the clinician’s request and the suspected illness. The suspected organisms were categorized as either bacteria, viral, fungi, and protozoan or a combination of bacteria/protozoan, fungi/protozoan, viral/protozoan, and viral/bacteria. Laboratory results confirmed only 4% of the cases with the majority being undetermined/not available/not known (96%, 937/978). The major organisms determined to be present include dengue virus, Staphylococcus aureus , Salmonella typhi , Streptococcus pneumonia , and Coxiella burnetii . a shows the absolute counts of each diagnosed organism coloured by the suspected organisms while b shows the proportion of each diagnosed organism coloured by the suspected organism. HIV stands for Human immunodeficiency virus, URTI for upper respiratory tract infection, Pf for Plasmodium falciparum and SPP for species
Haematological parameters vary between nMI, UM, and SM
Median values for all the haematological parameters were significantly different among nMI, UM, and SM ( P < 0.001) (Table 2 ), but most of the parameters do not show distinct distributions between the different clinical diagnosis groups (Fig. 3 ). More so, Dunn’s post hoc tests indicated that platelet distribution width, percentage neutrophils, and percentage lymphocytes were not significantly different between the nMI and SM (Table 2 ). Similarly, the pairwise comparisons showed that mean cell volume, neutrophil count, and mean platelet volume were not significantly different between nMI and UM (Table 2 ). Despite the statistical test, we hypothesized that the median differences for each parameter cannot be used to confidently classify the disease outcomes.
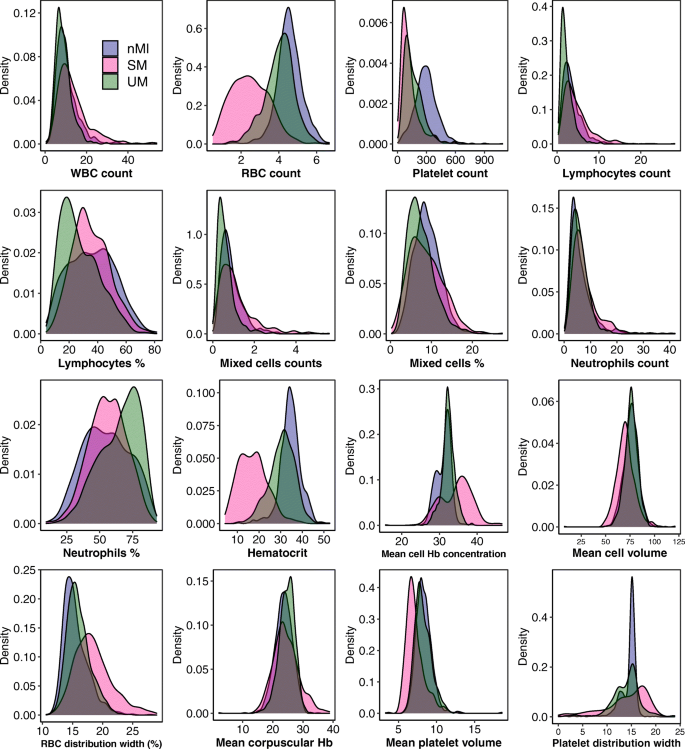
Density estimates of the haematological parameters between nMI, UM, and SM cases. The plots indicate the distribution of each haematological parameter for each clinical diagnosis category. The plot uses the kernel density estimate that allows for smoother distributions by smoothing out the noise. The peaks of each density plot are displaying the point where values are concentrated over the interval. Below each plot is the label of the haematological parameter it is estimating
To further confirm this hypothesis, the median was used to split the variables into categorical variables (low and high levels). The relationship or predictive value of the categorical parameters to accurately classify the clinical diagnosis was determined using contingency tables (Additional file 2 : Table S3). The percentage number of individuals who had low levels of each parameter and were classified with nMI ranged from 29 to 70% (UM group) and 7 to 82% (SM group) (Fig. 4 a). Comparatively, the percentage of individuals who had low levels of each parameter and were classified with UM ranged between 30 and 71%, while the percentage of individuals who were classified with SM ranged between 17 and 91% (Fig. 4 b). There were similar trends for the percentage number of individuals who had high levels of each parameter and were classified with either nMI, UM, or SM (Fig. 4 c, d).
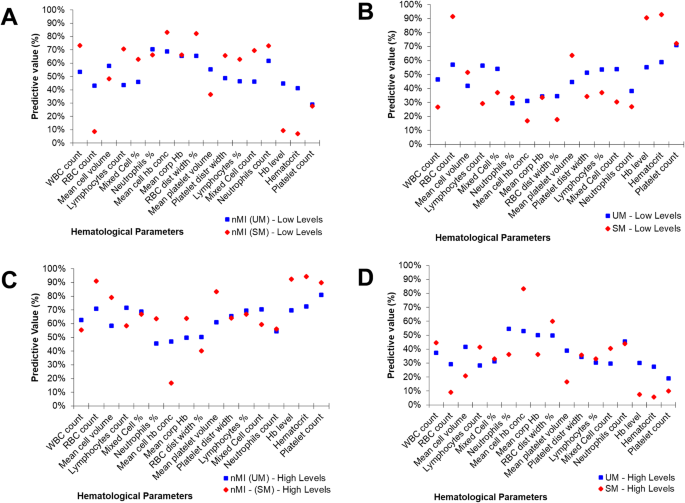
Non-symmetrical predictive values of clinical diagnosis using median split (high vs low levels) of each haematological parameter. A ‘median split’ was used to divide each quantitative parameter into categorical variables by the median value (calculated as a mean of nMI and UM or SM median value shown in Table 2 ). The predictive values are calculated from contingency tables (Additional file 2 : Table S3). a The percentage predictive value in predicting nMI from low levels. b Percentage predictive value in predicting SM or UM using the low levels. c Percentage predictive value in predicting nMI using high levels. d Predictive values of UM or SM using high levels
Additionally, we determined whether the levels could predict whether an individual has UM or SM using odds ratios. First, we predicted UM, and majority of the parameters were associated with clinical diagnosis of UM and nMI ( P < 0.001), except mean cell volume, lymphocyte percentage, mixed cell counts, and neutrophil counts (Additional file 1 : Table S4). The parameters that were not associated for nMI–SM category were lymphocyte counts, mean corpuscular Hb, lymphocyte percentage, mixed cell counts, and neutrophil counts (Additional file 1 : Table S4). Furthermore, some of the haematological parameters had a 95% confidence interval that included the null value (1) when evaluating the odds ratios, which signifies that they are not significantly associated with clinical diagnosis (Additional file 1 : Table S4).
Machine learning attained over 77.7% accuracy in classifying clinical malaria from nMI
Since there is no clear distinction between the distributions and the inability of the median-based categories to clearly classify the participant’s clinical diagnosis, we sought to evaluate six ML approaches to classify clinical malaria from nMI. The UM vs nMI model was trained on 942 samples, validated on 403 samples, and tested on 336 samples for each ML approach. The SM vs nMI model was trained on 843 samples, validated on 361 samples, and tested on 300 samples for each ML approach (Fig. 1 ). Among the six ML approaches, the training accuracies ranged between 0.794 and 0.856 to classify UM while the training accuracies ranged between 0.937 and 0.985 in classifying SM. The test accuracies ranged from 0.777 to 0.857 for the UM model and 0.930 to 0.973 for the SM model (Additional file 1 : Table S5). The SVM approach and the ANN generated the overall best classification outcome.
Hyperparameter tuning for the ANN ( n = 55,290 combinations) showed that the optimal model for multi-classification had 0.862 training accuracy with a model capacity of 3 layers (128, 64, and 16), with dropouts of 0.4 for layer 1, 0.3 for layer 2, and 0.4 for layer 3, and learning rate of 0.001 (as represented in Additional file 1 : Fig. S2). The optimal model ( n = 55,290 combinations) for ANN (nMI vs SM) with 0.985 accuracy had a model capacity of 3 layers (16, 128, and 256 RELU units, respectively), the dropout rate was 0.2 and 0.4 for the first two layers and the last layer had 0.1, and a learning rate of 0.0001. The optimal model ( n = 55,290 combinations) for ANN (nMI vs UM) with 0.856 training accuracy had a model capacity of 3 hidden layers of 256, 64, and 16 RELU units, respectively; the dropout rate was 0.1 for the first and last layer and 0.3 for the second layer, and a learning rate of 0.0001. Training and validation history plots for the ANN showed good levelling off for accuracy and loss, as well as acceptable divergence between training loss/accuracy and validation loss/accuracy for all the three models (Additional file 1 : Fig. S3).
Also, the history plots suggest that there was near zero over-fitting or under-fitting of the data as indicated by closeness of the training and validation curves (Additional file 1 : Fig. S3). The ANN (UM vs. nMI) achieved 0.856 training accuracy and 0.842 validation accuracy, while the testing accuracy of the model was 0.801 (kappa 0.583) (Table 3 ). The training and testing accuracies demonstrate the confidence of the networks in classifying UM. The ANN (SM vs nMI) achieved a higher accuracy (≥ 0.96) for training, validation, and testing accuracy (Table 3 ). Both ANN had an F1 score of above 0.747, which means the model can be used for the classification of clinical malaria (Table 3 ). Since the binary classifiers had the best performance, we also performed a multi-classification analysis to assess the ability of the ANN to differentiate among UM, SM, and nMI. The data available for the multi-classification model was 2,207 samples, which were split to 80% training ( n = 1,766) and 20% testing ( n = 441). The training data was further split to 70% ( n = 1,236) training and 30% ( n = 530) cross-validation with accuracies of 0.862 and 0.828, respectively. The test accuracy was 0.853; kappa, precision, recall, and F1 score of the model was > 0.768 (Table 3 ). The accuracy of multi-classification model provides confidence in the binary classifications.
Diagnostic value of the models using ROC curves
Having shown the accuracy of the models, we determined the ROC curves of ANN (UM vs nMI) and ANN (SM vs nMI) to show the diagnostic ability of these binary classifiers. Both classifiers had very good performance with an AUC of 0.866 for ANN (UM vs nMI) and AUC of 0.983 for ANN (SM vs nMI) (Fig. 5 and Table 3 ). This showed that the models could be used to distinguish individuals with SM or UM from those with nMI. The cut-offs for UM show that there is a trade-off in sensitivity and specificity as the cut-off increases or decreases, which is not the case for SM. These results could frame the clinical utility of the models and provide a benchmark for future studies.
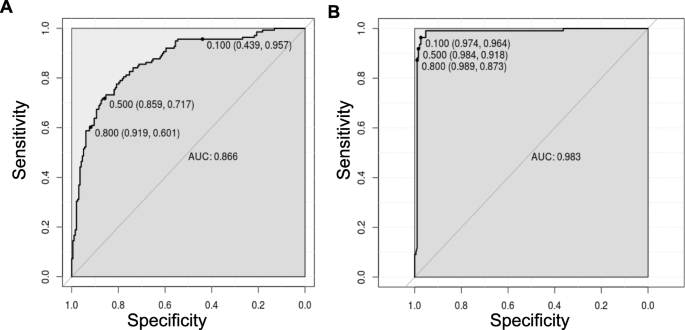
ROC curve for classification of SM was near perfect. The ROC curve plots sensitivity versus specificity for all possible cut-offs. Each point on the curve represents a different cut-off value, which is connected to form a curve. The diagonal line is a reference line for the ROC curve. a ROC for the ANN (UM vs nMI) with an area under the curve (AUC) of 0.866 which is basically an average of true positive rate across all possible false positive rates. b ROC for the ANN (SM vs nMI) is right angled which means its near perfect with an AUC of 0.983. The levels of AUC indicate a good performance of the models in classifying UM and SM
Platelet and RBC counts classify clinical malaria from non-malaria infections
The models were investigated to identify which haematological parameters were classified to be important for either SM, UM, or nMI using local interpretable model-agnostic explanations (LIME). Case by case analysis of the individuals showed that some haematological parameters are important classifiers of UM (Additional file 1 : Fig. S4). Case by case analysis was merged into heatmap to generate a consolidated picture of useful parameters for classification (Fig. 6 ). The top three parameters that had low feature weights for UM are platelet counts, RBC counts, and lymphocyte percentages (Fig. 6 a). Based on the order of importance, the top three parameters that were important for SM classification include RBC counts, platelet counts, and mean platelet volume (Fig. 6 b). This shows that both platelet and RBC counts are important parameters for clinical malaria while the lymphocyte percentages were unique for UM. These parameters might be used to classify clinical malaria cases from nMI, with a very good diagnostic ability as shown by the ROC analysis (Fig. 5 ).
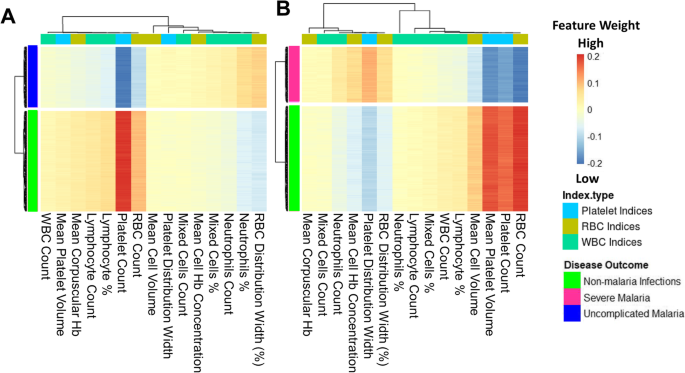
Platelet and RBC counts identified as classifiers of both UM and SM. The Keras model was explained using local interpretable model-agnostic explanations (LIME Package in R-software). The explainer results of the test data, which are represented as feature weights, were extracted from the explainer and used to plot the heatmaps to show a consolidated picture of the importance of each haematological parameter. The weights that are < − 0.1 indicate that they are low during UM or SM. a The heatmap shows that platelet, RBC, and lymphocyte percentages/counts can classify UM and b shows the haematological parameters that can classify SM, and they include RBC counts, platelet counts, mean platelet volume, and mean cell volume
Patient age and sampling location do not affect the model classifications
We further tested if the models are agnostic to age and location variance. There was a significant difference in patient age between nMI and UM ( P < 0.001), but there was no significant difference in samples within Kintampo as well as children under the age of 4 years (Fig. 7 a, c, e; Additional file 1 : Fig. S5 & S6). The performance accuracy of the random forest models was 0.806, 0.767, and 0.768 for models 1, 2, and 3, respectively (Fig. 7 b, d, f). The most important parameters that were featured across the three models were platelet and RBC counts, which are similar to the top two parameters identified by the ANN. Therefore, the data illustrates that age and location do not affect model classifications, and the platelet or RBC counts determined by ANN can be used to reliably classify clinical malaria from nMI in these datasets.
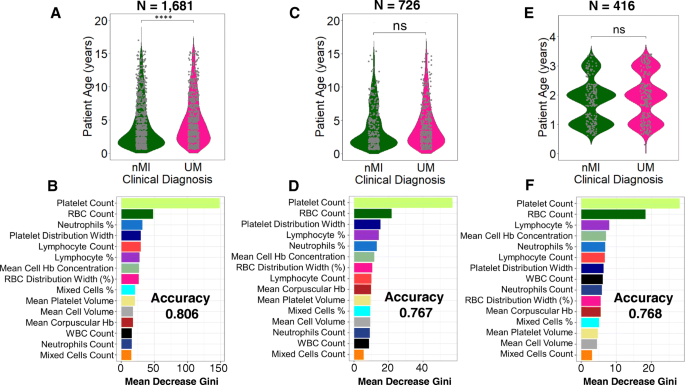
Classification of haematological parameters using random forest shows that patient age and sampling location do not affect the ML models. Three models were generated: a a model for all the UM and nMI cases ( n = 1681), which show a significant difference in patient age, while b shows the impurity-based measurement of the feature importance of the model; c a model for UM and nMI from Kintampo cases only ( n = 756), which do not show any significant difference between the patient age, and d shows the feature importance of the model; and e a model for only Kintampo cases and ages under 4 years, whereby there was no significant difference between the nMI and UM ( n = 416) and f shows the feature importance of the model. The samples for each model were split 80% for training and 20% for testing. The accuracy of the models was 0.806, 0.767, and 0.768, respectively. The most important feature across the three models was platelet and RBC counts
Automated CBC is one of the blood tests routinely performed for children presenting to health facilities with fever. However, CBC analysis generates a significant amount of data on a range of haematological parameters, and the data is underutilized with only Hb and Hct levels being routinely used as an indicator of clinical malaria. Thus, an automated algorithm to detect malaria based on the haematological parameters as outlined in this study could have great value as a complementary malaria diagnostic strategy, particularly at frontline health centres where CBC is routinely performed. Such an algorithm also has the added value of enabling the monitoring of treatment outcomes for in-patients.
In malaria-endemic settings, malaria rapid diagnostic tests (mRDTs) have revolutionized diagnosis and significantly reduced presumptive treatment, particularly in rural settings where trained microscopists are lacking [ 3 ]. However, reports of emerging Pfhrp2/3 gene deletions threaten the future reliability of the RDTs. False negative RDT results are also known to occur in low-density infections [ 2 , 6 , 7 ]. Thus, an approach that is automated and agnostic to parasite genetic variation is critical both as a fail-safe and a surveillance strategy for false negative mRDTs (which might occur due to supply chain mismanagement or gene variation) [ 6 ]. In very low transmission settings, ML models have the potential to replace the primary use of mRDTs when the diagnostic yield of mRDTs becomes very low (i.e. many mRDTs needed to detect a single case of malaria). In non-endemic settings where malaria may occur in immigrants and non-immune travellers, the models may allow another fail-safe mechanism in case the diagnosis of malaria was not suspected by clinicians and malaria RDT or microscopy was not performed. Despite these advantages, there would be a little extra cost associated with incorporating the algorithm and an automated message into haematology analyser output, a message that can prompt clinicians to consider malaria in the presence of suggestive haematological features.
Previous ML studies have looked into haematological parameters more generally and to classify sickle cell anaemia using deep convolutional networks [ 41 , 42 ], but did not classify clinical diagnosis. For the first time, ML approaches that can classify infections in children based on haematological parameters have been generated. Six different ML methods were evaluated, and they were all shown to classify clinical malaria from nMI with high accuracy especially the SVM and the ANN. We used the ANN to deconvolute the results: it identified platelet and RBC counts as the top features in classifying both UM or SM from nMI. Low RBC counts can be attributed to extensively parasitized RBCs, which are sequestered during SM [ 43 ]. This highlights the significance of RBC counts during Plasmodium falciparum malaria infections. In most occasions except cerebral malaria, SM is associated with anaemia due to RBC lysis during parasite invasion as well as many other RBC abnormalities [ 44 ]. This makes the diagnosis of SM much easier than UM, whereby one parameter, such as Hb level of < 5 g/dL, can diagnose or classify the disease.
Cohen et al. analysed data from 680,964 individuals with fever and confirmed that majority of antimalarial drugs are given to malaria-negative individuals [ 45 ]. Overtreatment indicates that most nMI can go without being treated, for their true cause, which is also possible for UM and this can lead to drug resistance. Therefore, the difference between febrile outpatient infections is far more challenging, especially between nMI and UM due to similarity in clinical presentations. In large population studies, values of studied metrics can be significant but they do not necessarily distinguish the populations as either nMI or UM as observed in this study. But, using the ML approach shown here, distinguishing the nMI and UM can be improved by combining all haematological parameters and learning the data-patterns before making classifications. The predictions made by ML are more accurate and reliable and can be improved by analysing more datasets. Lymphocyte counts/percentage were identified to be affected during UM and can be used to distinguish UM from nMI, mainly because individuals with malaria generally have a distinct immune response compared to nMI individuals [ 27 , 46 , 47 ].
Previous work in our laboratory showed differences in haematological presentation among areas of varying transmission intensity in Ghana [ 48 ]. To show that differences in age and transmission zones (sampling location) are not driving our diagnostic classifications, we down-sampled the data and used random forest to perform the classifications. The results showed that platelet and RBC counts were the key features in classifying UM and nMI regardless of age and sampling location of the participants. There were differences in the top three important features between the random forest and ANN, but this could be due to the differences in the approach of each algorithm [ 23 , 49 ]. This illustrates that patient age and location do not substantially influence the diagnostic classifications in this study. The ROC curves further showed that the models could be used for diagnosis with very reliable AUC values.
There are limitations to be considered in the use of this ML approach in routine diagnosis and the generalization of our approach. First, the models can distinguish between nMI and clinical malaria, but whether they can be used to distinguish the clinical disease state will depend on the pre-test probability or prevalence of malaria in different endemic settings. Second, all study subjects being Ghanaian children may limit the generalizability of the models to other countries; this is also the case for the limited range of SM manifestations in our dataset and the spectrum of laboratory-confirmed nMI. The few nMI cases that were clearly diagnosed and still grouped/retained as nMI may also present minimal bias to the models. Lastly, the study did not have adults > 15 years to comparatively understand the role of age in differentiating clinical malaria based on haematological parameters. Therefore, we recommend that more studies are needed to inform the broader utility of this work. Despite that only 4.6% (75/1645) of the cases were discordant between microscopy and RDT, probably due to hrp2/hrp3 deletions, although there is an insignificant chance that misclassification of malaria could have had an impact on our study. These limitations will be taken into account for further studies to inform the broader clinical utility of this work.
Conclusions
Fever is the most common symptom reported in sSA, and correct diagnosis of the implicated pathogen is of high importance for precision medicine. Personalized treatment reduces overtreatment, decreases malaria mortality and antimalarial resistance. This report demonstrates proof-of-principle that ML can be used to distinguish clinical malaria from nMI using routine haematological data. Case by case analysis showed that the models can make classifications based on combination of three parameters: platelet and RBC counts, lymphocyte counts/percentage, and mean platelet volume. These could be used for precision diagnosis of an individual’s risk of having malaria, to inform the need for confirmatory diagnosis by microscopy or to prompt investigation for other diagnoses when malaria is unlikely. Further work is to calibrate and improve the classification capability of the model using more data from other geographical and transmission settings, demographic groups, co-infections, and different disease severities. Our findings hold promise for the design of clinical software to support the diagnosis of malaria in the WHO African region and might also prove useful for the diagnosis of malaria in returning travellers from non-endemic countries.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
WHO. World malaria report; World Health Organization 2018;4:186. http://apps.who.int/iris/bitstream/10665/254912/1/WHO-HTM-GMP-2017.4-eng.pdf?ua=1 . Accessed 15 Oct 2017.
Watson OJ, Sumner KM, Janko M, Goel V, Winskill P, Slater HC, et al. False-negative malaria rapid diagnostic test results and their impact on community-based malaria surveys in sub-Saharan Africa. BMJ Glob Heal. 2019;4:e001582. https://doi.org/10.1136/bmjgh-2019-001582 .
Article Google Scholar
Mouatcho JC, Dean Goldring JP. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol. 2013;62:1491–505. https://doi.org/10.1099/jmm.0.052506-0 .
Article PubMed CAS Google Scholar
WHO. False-negative RDT results and P. falciparum histidine-rich protein 2/3 gene deletions. World Heal Organ. 2017. doi: https://doi.org/10.1186/1475-2875-10-166 .
Wanja EW, Kuya N, Moranga C, Hickman M, Johnson JD, Moseti C, et al. Field evaluation of diagnostic performance of malaria rapid diagnostic tests in western Kenya. Malar J. 2016;15:456. https://doi.org/10.1186/s12936-016-1508-y .
Article PubMed PubMed Central Google Scholar
Agaba BB, Yeka A, Nsobya S, Arinaitwe E, Nankabirwa J, Opigo J, et al. Systematic review of the status of pfhrp2 and pfhrp3 gene deletion, approaches and methods used for its estimation and reporting in Plasmodium falciparum populations in Africa: review of published studies 2010-2019. Malar J. 2019;18:355. https://doi.org/10.1186/s12936-019-2987-4 .
Article PubMed PubMed Central CAS Google Scholar
Ranadive N, Kunene S, Darteh S, Ntshalintshali N, Nhlabathi N, Dlamini N, et al. Limitations of rapid diagnostic testing in patients with suspected malaria: a diagnostic accuracy evaluation from Swaziland, a low-endemicity country aiming for malaria elimination. Clin Infect Dis. 2017;64:1221–7. https://doi.org/10.1093/cid/cix131 .
Angelo KM, Libman M, Caumes E, Hamer DH, Kain KC, Leder K, et al. Malaria after international travel: a GeoSentinel analysis, 2003–2016. Malar J. 2017;16:293. https://doi.org/10.1186/s12936-017-1936-3 .
Grobusch MP, Schlagenhauf P. Self-diagnosis and self-treatment of malaria by the traveler. Travel Med. 2019;:169–78. doi: https://doi.org/10.1016/B978-0-323-54696-6.00016-1 .
Lampah DA, Yeo TW, Malloy M, Kenangalem E, Douglas NM, Ronaldo D, et al. Severe malarial thrombocytopenia: a risk factor for mortality in Papua, Indonesia. J Infect Dis. 2015;211:623–34.
Hanson J, Phu NH, Hasan MU, Charunwatthana P, Plewes K, Maude RJ, et al. The clinical implications of thrombocytopenia in adults with severe falciparum malaria: a retrospective analysis. BMC Med. 2015;13:97. https://doi.org/10.1186/s12916-015-0324-5 .
White NJ. Anaemia and malaria. Malar J. 2018;17:371. https://doi.org/10.1186/s12936-018-2509-9 .
Squire DS, Asmah RH, Brown CA, Adjei DN, Obeng-Nkrumah N, Ayeh-Kumi PF. Effect of Plasmodium falciparum malaria parasites on haematological parameters in Ghanaian children. J Parasit Dis. 2016;40:303–11.
Article CAS Google Scholar
Muwonge H, Kikomeko S, Sembajjwe LF, Seguya A, Namugwanya C. How reliable are haematological parameters in predicting uncomplicated Plasmodium falciparum malaria in an endemic region? ISRN Trop Med. 2013;2013:1–9.
Anabire NG, Armah P, Francis A, Frank A, Osman A, Kanwugu N, et al. Evaluation of haematological indices of childhood illnesses in Tamale Metropolis of Ghana. 2018.
Google Scholar
Warimwe GM, Recker M, Kiragu EW, Buckee CO, Wambua J, Musyoki JN, et al. Plasmodium falciparum var gene expression homogeneity as a marker of the host-parasite relationship under different levels of naturally acquired immunity to malaria. PLoS One. 2013;8:e70467. https://doi.org/10.1371/journal.pone.0070467 .
Kotepui M, Phunphuech B, Phiwklam N, Chupeerach C, Duangmano S. Effect of malarial infection on haematological parameters in population near Thailand-Myanmar border. Malar J. 2014;13.
Kotepui M, Piwkham D, PhunPhuech B, Phiwklam N, Chupeerach C, Duangmano S. Effects of malaria parasite density on blood cell parameters. PLoS One. 2015;10..
Lee SJ, Stepniewska K, Anstey N, Ashley E, Barnes K, Binh TQ, et al. The relationship between the hemoglobin concentration and the haematocrit in Plasmodium falciparum malaria. Malar J. 2008;7.
Lecun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-44. https://doi.org/10.1038/nature14539 .
Schmidhuber J. Deep learning in neural networks: an overview. Neural Netw. 2015;61:85-117. https://doi.org/10.1016/j.neunet.2014.09.003 .
Mikolov T, Sutskever I, Chen K, Corrado G, and Dean J. Distributed representations of words and phrases and their compositionality. Proc Adv Neural Inf Process Syst. 2013;2:3111–9.
Mooney SJ, Pejaver V. Big data in public health: terminology, machine learning, and privacy. Annu Rev Public Health. 2018;39:95-112. https://doi.org/10.1146/annurev-publhealth-040617-014208 .
Camacho DM, Collins KM, Powers RK, Costello JC, Collins JJ. Next-generation machine learning for biological networks. Cell. 2018;173:1581–92. https://doi.org/10.1016/j.cell.2018.05.015 .
Parveen R, Jalbani AH, Shaikh M, Memon KH, Siraj S, Nabi M, et al. Prediction of malaria using artificial neural network. Int J Comput Sci Netw Secur. 2017;17:79–86.
Poostchi M, Silamut K, Maude RJ, Jaeger S, Thoma G. Image analysis and machine learning for detecting malaria. Transl Res. 2018;194:36. https://doi.org/10.1016/J.TRSL.2017.12.004 .
Bediako Y, Adams R, Reid AJ, Valletta JJ, Ndungu FM, Sodenkamp J, et al. Repeated clinical malaria episodes are associated with modification of the immune system in children. BMC Med. 2019;17:60. https://doi.org/10.1186/s12916-019-1292-y .
KalantarMotamedi Y, Eastman RT, Guha R, Bender A. A systematic and prospectively validated approach for identifying synergistic drug combinations against malaria. Malar J. 2018;17:160. https://doi.org/10.1186/s12936-018-2294-5 .
Shrinet J, Nandal UK, Adak T, Bhatnagar RK, Sunil S. Inference of the oxidative stress network in Anopheles stephensi upon Plasmodium infection. PLoS One. 2014;9:e114461. https://doi.org/10.1371/journal.pone.0114461 .
Thakur S, Dharavath R. Artificial neural network based prediction of malaria abundances using big data: a knowledge capturing approach. Clin Epidemiol Glob Heal. 2019;7:121–6. https://doi.org/10.1016/J.CEGH.2018.03.001 .
Butler KT, Davies DW, Cartwright H, Isayev O, Walsh A. Machine learning for molecular and materials science. Nature. 2018;559:547–55. https://doi.org/10.1038/s41586-018-0337-2 .
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Croatian Med J. 2003;44:635–8.
Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6:e012799.
Oduro AR, Koram KA, Rogers W, Atuguba F, Ansah P, Anyorigiya T, et al. Severe falciparum malaria in young children of the Kassena-Nankana district of northern Ghana. Malar J. 2007;6:96.
World Health Organization (WHO). Management of severe malaria: a practical handbook. 3rd edition. WHO Library Cataloguing-in-Publication Data; 2013.
Iacobucci D, Posavac SS, Kardes FR, Schneider MJ, Popovich DL. The median split: robust, refined, and revived. J Consum Psychol. 2015;25:690–704.
Stephen Milborrow. Derived from mda:mars by Trevor Hastie and Rob Tibshirani. Uses Alan Miller’s Fortran utilities with Thomas Lumley’s leaps wrapper. earth: Multivariate Adaptive Regression Splines version 5.1.2 from CRAN. https://rdrr.io/cran/earth/ . Accessed 24 Aug 2020.
Quintó L, Aponte JJ, Menéndez C, Sacarlal J, Aide P, Espasa M, et al. Relationship between hemoglobin and haematocrit in the definition of anaemia. Trop Med Int Heal. 2006;11:1295–302.
Ribeiro MT, Singh S, Guestrin C. “Why should I trust you?” Explaining the predictions of any classifier. Scand J Infect Dis. 2016;46:1135–44.
Liaw A, Wiener M. Classification and regression by randomForest. 2002. http://www.stat.berkeley.edu/ . Accessed 12 May 2020.
Xu M, Papageorgiou DP, Abidi SZ, Dao M, Zhao H, Karniadakis GE. A deep convolutional neural network for classification of red blood cells in sickle cell anemia. PLoS Comput Biol. 2017;13:e1005746. https://doi.org/10.1371/journal.pcbi.1005746 .
Gunčar G, Kukar M, Notar M, Brvar M, Černelč P, Notar M, et al. An application of machine learning to haematological diagnosis. Sci Rep. 2018;8:411. https://doi.org/10.1038/s41598-017-18564-8 .
White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet (London, England). 2014;383:723–35. https://doi.org/10.1016/S0140-6736(13)60024-0 .
Akinosoglou KS, Solomou EE, Gogos CA. Malaria: a haematological disease. Hematology. 2012;17:106–14. https://doi.org/10.1179/102453312X13221316477336 .
Cohen JM, Woolsey AM, Sabot OJ, Gething PW, Tatem AJ, Moonen B. Optimizing investments in malaria treatment and diagnosis. Science (80-). 2012;338:612–4. https://doi.org/10.1126/science.1229045 .
Kurup SP, Butler NS, Harty JT. T cell-mediated immunity to malaria. Nat Rev Immunol. 2019;19:457–71. https://doi.org/10.1038/s41577-019-0158-z .
Ly A, Hansen DS. Development of B cell memory in malaria. Front Immunol. 2019;10:559. https://doi.org/10.3389/fimmu.2019.00559 .
Mensah-Brown HE, Abugri J, Asante KP, Dwomoh D, Dosoo D, Atuguba F, et al. Assessing the impact of differences in malaria transmission intensity on clinical and haematological indices in children with malaria. Malar J. 2017;16:96. https://doi.org/10.1186/s12936-017-1745-8 .
Godfellow I, Bengio Y, Courville A. Deep learning. 2016.
CDC. Clinical reference ranges. 2013.
Download references
Acknowledgements
We acknowledge the study participants as well as the staff of Kintampo Municipal, who provided support for this study. We are grateful to Emmanual Allotey, Prince Nyarko, Henrietta Mensa-Brown, Felix Ansah, Jersley Chirawurah, Jonas Kengne, Nsoh Godwin Anabire, Reuben Ayivor-Djanie, and Nancy Nyakoe for their contributions on the data collection and constructive criticism of the work. We also express our sincere gratitude to Dorothy Annan, Deborah Mettle, Bright Yemi, Rachel Abban, Samirah Saiid, Joyceline Kwarko, and Israel Osei for assisting in cross-referencing the data. We acknowledge the University of Ghana for providing the high-performance computing resources (the ZUPUTO) used for this work.
The study was supported by a DELTAS Africa grant (DEL-15-007: Awandare). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107755/Z/15/Z: Awandare) and the UK government. TO is supported by the Wellcome Trust grant 104111/Z/14/ZR. The funder had no role in the study design and where to publish. The views expressed in this publication are those of the authors and not necessarily those of AAS, NEPAD Agency, Wellcome Trust, or the UK government.
Author information
Authors and affiliations.
West African Centre for Cell Biology of Infectious Pathogens (WACCBIP), Department of Biochemistry, Cell and Molecular Biology, University of Ghana, Legon, Accra, Ghana
Collins M. Morang’a, Lucas Amenga–Etego, Saikou Y. Bah, Vincent Appiah, Dominic S. Y. Amuzu, Nicholas Amoako & Gordon A. Awandare
Florey Institute, Molecular Biology and Biotechnology, University of Sheffield, Sheffield, UK
Saikou Y. Bah
Department of Applied Chemistry and Biochemistry, C. K Tedam University of Technology and Applied Sciences, Navrongo, Ghana
James Abugri
Ministry of Health, Navrongo Health Research Centre (NHRC), Navrongo, Ghana
Abraham R. Oduro
Section of Pediatric Infectious Disease, Department of Infectious Disease, Imperial College London, London, UK
Aubrey J. Cunnington
Institute of Infection, Immunity & Inflammation, MVLS, University of Glasgow, Glasgow, UK
Thomas D. Otto
You can also search for this author in PubMed Google Scholar
Contributions
CM, GA, LA, TO, SB, DA, AC, and VA contributed to the design and conceptualization of the work, as well as editing and critique of the manuscript drafts. LA, JA, DA, AO, and NA contributed to the acquisition of data. CM performed the data analysis, including building the models, model interpretation, and drafting the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Lucas Amenga–Etego or Thomas D. Otto .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committees of the Ghana Health Service (GHS-ERC: 12/05/12 & GHS-ERC 12/06/16), the Kintampo Health Research Centre (KHRCIEC/FEA/2011-13), the Navrongo Health Research Centre (NHRC-IRB135/08/2012), and the Noguchi Memorial Institute for Medical Research (NMIMR) (NMIMR-IRB CPN 004/11-12). All participants were provided with written informed consent prior to inclusion in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
The list of haematological parameters adopted from laboratory procedure manual by the CDC [ 50 ]. Table S4. The odds ratio of median categories providing the odd of being diagnosed with either nMI, UM, and SM. The median categories were; low and high levels. Table S5 . Performance evaluation of six machine learning models to classify clinical malaria outcomes. Fig. S1 . Word cloud of clinical manifestations using clinicians/doctors notes or suspected infections. Fig. S2. Artificial Neural Network Schematic. Fig. S3. Plot for the training and validation history of the ANN. Fig. S4. Case by case analysis of the classification capability of the ML models. Fig. S5. Density estimates of the haematological parameters between nMI and UM cases for sub-sampled data from Kintampo only. Fig. S6. Density estimates of the haematological parameters between nMI, and UM cases for sub-sampled data from Kintampo only, as well limit of children under 4 years of age.
Additional file 2: Table S2
. Clinical and raw haematological data of study participants. Table S3. The median splits predictive values on the clinical diagnostic categories (nMI, UM, and SM) of the study participants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Morang’a, C.M., Amenga–Etego, L., Bah, S.Y. et al. Machine learning approaches classify clinical malaria outcomes based on haematological parameters. BMC Med 18 , 375 (2020). https://doi.org/10.1186/s12916-020-01823-3
Download citation
Received : 24 June 2020
Accepted : 22 October 2020
Published : 30 November 2020
DOI : https://doi.org/10.1186/s12916-020-01823-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Uncomplicated Malaria
- Severe Malaria
- Haematological parameters
- Classification
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

The answer to your search may depend on where you live
Putting human past on the MAPS

Does AI help humans make better decisions?
Tracking entwined histories of malaria, humans.

Upper Mustang region of Nepal.
Photo by Christina Warinner
Christy DeSmith
Harvard Staff Writer
New study of ancient genomes tracks disease over 5,500 years, factors in spread, including trade, warfare, colonialism, and slavery
Today, Malaria represents a major public health problem across much of the globe, killing more than 600,000 people annually and infecting another 250 million. It is a disease that has been around for millions of years and is undeniably entwined with human history.
“Malaria has actually shaped the human genome,” said Megan Michel , a Ph.D. candidate in human evolutionary biology at the Harvard Kenneth C. Griffin Graduate School of Arts and Sciences . She pointed out that certain inherited blood disorders, like sickle cell disease, rose in prevalence because they provide a measure of resistance to the mosquito-borne infection.
Now a new study led by Michel reconstructs the ancient genomes of the two deadliest malaria parasites, Plasmodium falciparum and Plasmodium vivax, with an eye to understanding the pathogen’s past. The research, published this week in Nature , tracks the disease over 5,500 years, with trade, warfare, colonialism, and slavery identified as major factors in its global spread.
The findings represent a feat of collaboration and data-sharing, with 94 co-authors representing 80 institutions and 21 countries. The DNA itself was plucked from genetic sequences collected from more than 10,000 ancient humans, with Michel identifying 36 malaria-infected individuals from 26 archaeological sites on five continents.
“For a graduate student to be coordinating all of this is really, really impressive,” said co-author Christina Warinner , the John L. Loeb Associate Professor of the Social Sciences and one of Michel’s three advisers. “By reconstructing these ancient Plasmodium genomes and comparing the genetic relationships between ancient and modern parasites, we’re finally able to place malaria in its evolutionary and human history context.”
“By reconstructing these ancient Plasmodium genomes and comparing the genetic relationships between ancient and modern parasites, we’re finally able to place malaria in its evolutionary and human history context.” Christina Warinner, the John L. Loeb Associate Professor of the Social Sciences
Malaria is marked by cyclical fevers that repeat every 48 or 72 hours. Until recently, written records were the only way researchers could track the disease’s progression across time and space. “There are descriptions in Greek and Roman texts that point to the presence of malaria,” Michel said. “But we were able to go back even further than that to show that malaria has been present in Europe for a very, very long time.”
The disease was also common in the U.S. until the arrival of modern drainage and insecticides in the 20th century. Warinner, a biomolecular archaeologist, pointed to the high number of U.S. presidents to suffer from malaria, including George Washington, Abraham Lincoln, and Ulysses S. Grant. “Teddy Roosevelt and JFK became infected while traveling,” she said, “but earlier presidents contracted it in their hometowns or in the Washington, D.C., area” — which was notoriously swampy.
The new paper features three compelling case studies, each illustrating the role of mobility in circulating malaria. The first concerns a Belgian cemetery , excavated between 2009 and 2011 and adjacent to the first permanent military hospital in early modern Europe. Historical records document that the Habsburg Army of Flanders recruited its soldiers from the Mediterranean region for its 80 Years’ War against Spain (1568-1648).
Malaria leaves no visible trace in human skeletal remains, but recent technological advances have enabled scientists to extract DNA from scraps of the pathogen found in teeth. Researchers were able to sequence malaria DNA from 10 individuals buried at the cemetery while also analyzing the genomes of soldiers who had been infected.
“We found that individuals buried at the cemetery have diverse ancestry profiles,” explained Michel, whose Ph.D. research is supported by the Max Planck - Harvard Research Center for the Archaeoscience of the Ancient Mediterranean . “They’re not just from Belgium. They seem to also be coming from northern Spain and from Italy.”
The two most prevalent malaria parasites in humans are P. vivax and P. falciparum, with the latter limited to warm climates and causing a more severe form of the disease. Analyses of pathogen DNA turned up a couple of P. vivax cases in the Belgian site’s local population, buried at the cemetery prior to the hospital’s construction in the mid-16th century.
Six cases, including several of the more virulent P. falciparum, were found in non-local individuals, all interred following the military hospital’s construction. Malaria cannot be transmitted through human contact, but mosquitos may have picked up these infections — and kept up the spread from there. “It’s even possible they ignited a local outbreak,” Michel said.

A bite from an infected mosquito transmits malaria.
Liz Zonarich/Harvard Staff; source: Mayo Clinic
The parasites travel to your liver where they lie dormant, usually about 10 days to four weeks.

Parasites leave the liver and infect red blood cells. Malaria signs and symptoms typically develop.

Malaria is transmitted to an uninfected mosquito when it bites someone with the disease.
Another case study came from Peru, where a single P. vivax case was found in a person who lived at high altitude (more than 9,300 feet) in the Central Andes. “This individual was associated with the Chachapoya culture ,” Michel said, “and the site we were working with spanned the period of European contact.”
For years, scientists have debated how the disease arrived in the Americas, where Indigenous populations lack genetic resistance to malaria. Reconstructing the genome of the Peruvian parasite revealed striking similarities to P. vivax strains found throughout South America today. It also resembled strains circulating in Europe during the 15th and 16th centuries.
“We think this is evidence that the species was transmitted by European colonizers to the Americas,” Michel said.
No ancient P. falciparum was found in the Americas, Michel noted, and P. falciparum strains circulating there today bear little resemblance to the ancient European P. falciparum parasites recovered by Michel and her co-authors. “Instead, American strains today look very similar to strains in Sub-Saharan Africa,” Michel said. “It seems likely that P. vivax was transmitted from Europe, whereas P. falciparum was probably transmitted from Sub-Saharan Africa as a result of the trans-Atlantic slave trade.”
Michel got her biggest surprise from the paper’s third case study. The Himalayan site of Chokhopani , situated more than 9,100 feet above sea level in Nepal’s Mustang region, yielded the earliest known case of P. falciparum .
“It’s the last place on Earth I would expect to find a malaria infection,” Michel shared. “It’s rocky and dry and too cold for malaria-transmitting mosquitoes to survive.”
The infected individual lived 2,800 years ago. “We know from the archaeological record that there was extensive long-distance trade in the region,” explained Michel, who partnered with co-author Mark Aldenderfer — an archaeologist working in the Mustang region for many years — to analyze the findings. “We think this was probably an individual who moved from low to high altitude, possibly for trade. They must have acquired this infection at a lower altitude where the parasite can be transmitted.”
“The site of Chokhopani is near the Kora La pass, the lowest crossing point through the Himalayas and a key trade route connecting South Asia with the Tibetan Plateau,” added Warinner, who traveled with Michel to the region last spring to share results and solicit feedback from descendent communities. “Fortunately, Nepal has been really successful in eradicating malaria in the last few years. But even as recently as 10 years ago, malaria was endemic in Nepal’s lower elevation regions.”
Making these revelations possible are the emerging tools of metagenomics , which rely on recovering and sharing as much genetic data as possible with different specialists. “When we analyze an ancient sample, we, by its nature, destroy it in order to retrieve the DNA,” Warinner explained. “We want to get as much information as possible. We really do recover total DNA.”
“Metagenomics and data-sharing allow us to find things we’re not really looking for,” Michel added. “It lets us find disease in unexpected places. I never would have screened samples from Chokhopani for malaria if they hadn’t already been sequenced by Dr. Warinner for another ancient DNA study.”
The research described in this report received funding from the National Science Foundation
Share this article
You might like.
Researchers find ‘language bias’ in various site algorithms, raising concerns about fallout for social divisions among nations

Harvard digital atlas plots patterns from history ancient and modern

One judge’s track record — with and without algorithm — surprises researchers
What the judge was thinking and what’s next in Trump documents case
Obama-era White House counsel says key point in Nixon decision should have ended inquiry
The way forward for Democrats — and the country
Danielle Allen is more worried about identity politics and gaps in civic education than the power of delegates
Finding right mix on campus speech policies
Legal, political scholars discuss balancing personal safety, constitutional rights, academic freedom amid roiling protests, cultural shifts
share this!
July 23, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
The unintended consequences of success against malaria
by North Carolina State University

For decades, insecticide-treated bed nets and indoor insecticide spraying regimens have been important—and widely successful—treatments against mosquitoes that transmit malaria, a dangerous global disease. Yet for a time, these treatments also suppressed undesirable household insects like bed bugs, cockroaches and flies.
Now, a new North Carolina State University study reviewing the academic literature on indoor pest control shows that as the household insects developed resistance to the insecticides targeting mosquitoes, the return of these bed bugs, cockroaches and flies into homes has led to community distrust and often abandonment of these treatments—and to rising rates of malaria.
The work appears in Proceedings of the Royal Society B: Biological Sciences .
In short, the bed nets and insecticide treatments that were so effective in preventing mosquito bites —and therefore malaria—are increasingly viewed as the causes of household pest resurgence.
"These insecticide-treated bed nets were not intended to kill household pests like bed bugs, but they were really good at it," said Chris Hayes, an NC State Ph.D. student and co-corresponding author of a paper describing the work. "It's what people really liked, but the insecticides are not working as effectively on household pests anymore."
"Non-target effects are usually harmful, but in this case they were beneficial," said Coby Schal, Blanton J. Whitmire Distinguished Professor of Entomology at NC State and co-corresponding author of the paper.
"The value to people wasn't necessarily in reducing malaria, but was in killing other pests," Hayes added. "There's probably a link between use of these nets and widespread insecticide resistance in these house pests, at least in Africa."
The researchers add that other factors—famine, war, the rural/city divide, and population displacement, for example—also could contribute to rising rates of malaria.
To produce the review, Hayes combed through the academic literature to find research on indoor pests like bed bugs, cockroaches and fleas, as well as papers on malaria, bed nets, pesticides and indoor pest control. The search yielded more than 1,200 papers, which—after an exhaustive review process—were whittled down to a final count of 28 peer-reviewed papers fulfilling the necessary criteria.
One paper—a 2022 survey of 1,000 households in Botswana—found that while 58% were most concerned with mosquitoes in homes, more than 40% were most concerned with cockroaches and flies.
Hayes said a recent paper—published after this NC State review was concluded—showed that people blamed the presence of bed bugs on bed nets.
"There is some evidence that people stop using bed nets when they don't control pests," Hayes said.
The researchers say that all hope is not lost, though.
"There are, ideally, two routes," Schal said. "One would be a two-pronged approach with both mosquito treatment and a separate urban pest management treatment that targets pests. The other would be the discovery of new malaria -control tools that also target these household pests at the same time. For example, the bottom portion of a bed net could be a different chemistry that targets cockroaches and bed bugs .
"If you offer something in bed nets that suppresses pests, you might reduce the vilification of bed nets."
Journal information: Proceedings of the Royal Society B
Provided by North Carolina State University
Explore further
Feedback to editors
'Miracle' filter turns store-bought LEDs into spintronic devices
7 hours ago

Climate-smart coffee: Researchers explore Robusta coffee as alternative to Arabica

Parched Central Valley farms depend on Sierras for groundwater

Researchers explore interplay between high-affinity DNA and carbon nanotubes

Perseverance rover discovers rock with potential signs of ancient life
8 hours ago

Fermi Telescope finds new feature in brightest gamma-ray burst yet seen
9 hours ago

Confined water gets electric: Study reveals dielectric response of water in nanopores

Underground CO₂ storage: Researchers measure carbon mineralization at unprecedented small scale

Climate change will bring more turbulence to flights in the Northern Hemisphere, analysis finds
10 hours ago

Liquid metals offer potential for greener chemical processes, researchers say
Relevant physicsforums posts, rock on mars possibly indicating life.
2 hours ago
The predictive brain (Stimulus-Specific Error Prediction Neurons)
Jul 21, 2024
Contradictory statements made by two different professors
The cass report (uk).
Jul 19, 2024
Understanding COVID Quarantine Guidance
Innovative ideas and technologies to help folks with disabilities.
Jul 18, 2024
More from Biology and Medical
Related Stories

New nets 'prevent' 13 mn malaria cases in Africa: Project
Apr 17, 2024

Pyrethroid plus bed nets reduce malaria infection in areas of high resistance
May 24, 2021
Research informs WHO mosquito net guideline update
Aug 16, 2023

Bed bugs modify microbiome of homes they infest
Jul 15, 2020

New research shows poor insecticide policy led to countless needless malaria cases
Jan 24, 2023

Changing the color of commonly used agricultural nets lessens insect damage to Kujo leek fields
Feb 14, 2024
Recommended for you

Your world is different from a pigeon's—but a new theory explains how we can still live in the same reality
12 hours ago

Does fertility affect a woman's body odor? Study finds no evidence
13 hours ago

Size doesn't matter for mammals with more complex brains, according to new study

New Zealand's flightless birds are retreating to moa refuges
Study reveals close host–symbiont interactions in deep-sea chemosynthetic tubeworm
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Open access
- Published: 06 October 2022
Severe malaria
- Nicholas J. White 1 , 2
Malaria Journal volume 21 , Article number: 284 ( 2022 ) Cite this article
25k Accesses
36 Citations
8 Altmetric
Metrics details
Severe malaria is a medical emergency. It is a major cause of preventable childhood death in tropical countries. Severe malaria justifies considerable global investment in malaria control and elimination yet, increasingly, international agencies, funders and policy makers are unfamiliar with it, and so it is overlooked. In sub-Saharan Africa, severe malaria is overdiagnosed in clinical practice. Approximately one third of children diagnosed with severe malaria have another condition, usually sepsis, as the cause of their severe illness. But these children have a high mortality, contributing substantially to the number of deaths attributed to ‘severe malaria’. Simple well-established tests, such as examination of the thin blood smear and the full blood count, improve the specificity of diagnosis and provide prognostic information in severe malaria. They should be performed more widely. Early administration of artesunate and broad-spectrum antibiotics to all children with suspected severe malaria would reduce global malaria mortality.
Severe malaria is important. It is a major cause of preventable childhood death in tropical countries. This large number of avoidable deaths justifies the substantial global investments in malaria control and elimination. But severe malaria is increasingly overlooked by the international agencies, donors and policy makers who determine the direction and support for global malaria initiatives.
Severe malaria, or ague, was recognized long before discovery of the malaria parasite by Laveran in 1880. The Cinchona bark arrived in Europe nearly four hundred years ago providing, for the first time, a potential cure for the pervasive and dangerous illness that then affected most of the inhabited world. But, as today, the specificity of the clinical diagnosis of febrile illnesses was poor. Torti recognized that only some fevers could be cured by the bark [ 1 ]. Even after the malaria parasite was identified first in 1880, severe forms such as algid malaria (shock), haemorrhagic or gastrointestinal malaria bore an uncertain relationship to Plasmodium infection, as did the notorious “blackwater fever”. Until the 1980s, the majority of research on severe malaria was conducted in adults. It derived largely from war-time experiences in the military, or observations from colonial medical services. Specific anti-malarial treatment comprised the parenteral administration of quinine and, from the 1950s, chloroquine. When they became available, renal replacement therapies for adult patients with acute renal failure could also save lives [ 2 ].
Soon after Laveran’s discovery of the causative parasite, the pathological processes underlying severe malaria were elucidated by the great Italian malariologists Marchiafava and Bignami. They considered, correctly, that the sequestration of parasitized erythrocytes in the microvasculature, causing microcirculatory dysfunction, was the key pathological event in “malignant tertian” (severe falciparum) malaria [ 3 , 4 ]. Beginning in the 1960s, coincident with the emergence of immunology as a discipline, and continuing to this day, various novel theories of severe malaria pathogenesis were proposed. These were often derived from observations in a murine “model” of cerebral malaria, which was fundamentally different to the human infection [ 2 , 5 ]. These new theories spawned a long succession of putative adjuvant therapies for severe malaria. Unfortunately, none of these therapies worked, and several were harmful [ 2 , 4 , 5 ].
1985 WHO meeting
Before 1985, there was no standard definition of severe malaria. Cerebral malaria was defined as unrousable coma (no localizing response to a painful stimulus). After publication of the Glasgow Coma Scale (GCS) in 1976 [ 6 ], this level of coma became a GCS less than 11. In 1985 an “informal meeting” was convened by the Malaria Action Programme of the World Health Organization (WHO). It was held in the Institute for Medical Research in Kuala Lumpur where, decades before, Field and colleagues had conducted seminal studies on the diagnosis, pathology and prognosis of severe malaria. The WHO meeting had the objective of reviewing available information on severe falciparum malaria, standardizing the definition, and advising on management [ 7 ]. The resulting document, which derived heavily from studies in Thailand conducted in the previous five years [ 8 ], provided a definition of severe malaria which is broadly similar to that used today, but with the following exceptions.
hyperparasitaemia was defined as > 5% parasitaemia (today this is 10%)
after a convulsion, coma had to persist for 6 h (now 30 min),
severe anaemia was defined as a haematocrit < 20% (now < 15%),
jaundice (total bilirubin > 50 µmol/L) alone was a criterion (today this requires a parasite density > 100,000/uL as well),
‘fluid, electrolyte or acid–base disturbances requiring intravenous therapy’ was a criterion (today more specific criteria have been instituted: either a venous plasma lactate > 5 mmol/L, arterial pH < 7.25, or a plasma bicarbonate < 15 mmol/L is required).
Hyperpyrexia (> 39 °C), vomiting of oral treatment and haemoglobinuria were also included – none of which today are considered defining criteria.
These definitions and descriptions have been generally referred to and referenced as “WHO definitions” although each successive version of the severe malaria review contains a disclaimer that the contents are the opinions of experts, and not those of the WHO itself.
1988 WHO meeting
In 1988 a second informal WHO meeting was held to update the recommendations and to incorporate recent observations in African children with cerebral malaria [ 9 ]. For the definition of severe malaria, hyperparasitaemia, jaundice, and hyperpyrexia were “dropped”, the haematocrit criterion was reduced to 15% and, after some debate, a requirement for a concomitant parasitaemia of > 10,000/µL was added to the severe anaemia criterion. Acidaemia or acidosis were defined as above, and repeated generalized convulsions (more than two observed within 24 h despite cooling) was added as a criterion. In this second meeting, the readily evaluated Blantyre Coma scale [ 10 ] was endorsed as the method to assess the level of consciousness in children.
1995 WHO meeting
The third WHO meeting was held in Geneva in December 1995 to incorporate further experience from clinical research in African children [ 10 – 12 ]. This meeting resulted in a broader, more inclusive and pragmatic, definition of severe malaria in children centred around prostration and respiratory distress (acidotic breathing) [ 13 ].
The hyperparasitaemia threshold was changed to 4% in low transmission settings, and to 20% in high transmission settings. The newly added “prostrate” criterion was very broad. It included many children with acute malaria who had no other signs of severity. This substantially expanded definition of severe malaria therefore encompassed a larger proportion of all children with acute malaria (and so it had a lower case-specific mortality). The new inclusive definition ensured a high proportion of at-risk children would be managed appropriately (i.e. it had high diagnostic sensitivity), but it had low specificity in identifying potentially fatal infections. In clinical research use of the broader inclusion criteria obviously resulted in overall “better outcomes” as more children with a good prognosis were included within the broader definition of severe malaria. Recognizing the disparities with the earlier criteria some investigators continued with the stricter (i.e. more specific) earlier severe malaria criteria [ 9 ] in their clinical research studies (Table 1 ).
2013 WHO meeting
The most recent WHO meeting on severe malaria was convened in 2013, again in Geneva [ 2 ]. By 2013, large prospective series of patients with severe malaria had been studied in Asia and Africa. These studies provided a much larger evidence base than for previous meetings. Many of the data came from randomized controlled trials [ 14 – 21 ]. The key therapeutic advance was the replacement of quinine by artesunate, which had been shown to reduce mortality by between one fifth and one third in very large randomized controlled trials [ 18 , 19 ]. The definitions of severe malaria, and components of the definitions, could now be associated with mortalities [ 22 – 26 ] (which were falling globally as artemisinin combination treatments were rolled out and parenteral artesunate was replacing quinine as first line parenteral treatment) [ 2 , 27 ]. The 2013 “WHO” meeting recognized both the requirements of a definition for practitioners, for whom sensitivity in recognizing potentially severe malaria and thus inclusiveness takes priority, with the contrasting needs of epidemiology and research studies where specificity is more important. The most recent research definition is shown in Table 2 [ 2 ].
Prostration was not included in the ‘research” definition, convulsions were “dropped”, the acidosis criterion was refined, the jaundice criterion was reintroduced with a parasite density > 100,000/µL, and the hyperparasitaemia criterion was changed (again!). In addition, severe Plasmodium vivax and Plasmodium knowlesi infections were reviewed specifically, and slightly modified definitions for severe malaria with these infections were proposed [ 2 ].
The meaning of severe malaria
Strictly speaking, severe malaria is malaria with an increased risk of death at the time of assessment compared to everyone else in that community with malaria illness. How much higher this risk should be (i.e. the lower threshold for the increase in mortality) has not been agreed upon. Mortality varies substantially as it depends on the infection, the host, the circumstances and the treatment. For the same admission severity, outcomes in well-equipped intensive care units (ICUs) with well-trained staff are better than in peripheral health centres. However, tertiary ICUs often receive the very sickest patients, often after long delays in referral – with consequently high mortalities. A frail and debilitated patient may die from a malaria infection that would be regarded as mild in a younger and fitter person. The high mortality of imported malaria (both P. falciparum and P. vivax ) in elderly travellers, and of malariatherapy (all species) in neurosyphilis, testifies to the lethal potential of acute malaria illness, whichever the infecting parasite species, in frail or debilitated persons [ 28 , 29 ]. In contrast, most people with malaria illness in endemic areas are either children or young adults without underlying conditions (although in Southern Africa HIV prevalence is high, and the untreated coinfection predisposes to severe malaria [ 30 ]). Acknowledging that this is an oxymoron, the “uncomplicated falciparum malaria” mortality of orally treated patients ranges from 1 in 10,000 to 1 in 1,000 if effective anti-malarial drugs are being used. Many factors affect this risk. Severe malaria usually has a mortality well over 5%, and therefore represents a > 50 fold increase in the risk of death. In general, as with many infections, mortality in malaria is proportional to the total number of infecting organisms (biomass) in the body. In non-immune adults mortality increases steeply as peripheral blood parasite densities rise over 100,000/µL [ 31 ]. This corresponds approximately to total parasite numbers within the blood of over 10 12 . If severe malaria was defined as clinical and laboratory measures which are associated with > 5% mortality, then the current thresholds would conform, except for the anaemia criterion (see below) which would require a threshold of 3 g/dL rather than 5 g/dL.
Malaria parasite densities
Malaria is traditionally diagnosed by microscopy examination of a peripheral blood smear. Unfortunately, this diagnostic skill is being lost in many places as microscopy is replaced by the more ‘convenient’, but less informative, rapid diagnostic tests. In malaria microscopy, the parasites are speciated and their numbers counted. The result is reported either as the number of parasitized erythrocytes in a stained thin smear or, in a thick film, as the number of parasites seen in a fixed volume or while counting a certain number of white blood cells (usually 200 or 500). The old semi-quantitative ‘cross’ system, in which density is graded from + to + + + + , is no longer recommended. The thin film should be used for high parasite densities (> 0.2% parasitaemia).
In falciparum malaria the parasite count can be misleading. This is because after approximately 12 to 16 h (depending on core temperature) of intraerythrocytic parasite growth (i.e. one quarter to one third of the asexual life cycle) Plasmodium falciparum infected erythrocytes begin to stick (“cytoadhere”) to vascular endothelium. By 20 h the majority have cytoadhered. This “sequestration” is the fundamental pathological process in falciparum malaria [ 2 , 3 ]. It occurs in all P. falciparum infections, although the tissue distribution of sequestration varies between patients. As a result, the parasite densities measured in blood films (reflecting circulating parasites only) variably underestimate the total malaria parasite biomass [ 32 – 34 ]. Nevertheless, the mortality of falciparum malaria is still proportional approximately to the peripheral blood parasite density. Among several factors, the relationship between peripheral blood parasite density and mortality depends on the prevailing intensity of transmission and thus the levels of “immunity” or “premunition”. Field showed in Kuala Lumpur (a generally low transmission area from the 1930s to the 1950s) that the mortality of falciparum malaria in adults with little or no immunity increased markedly when parasite densities rose above 100,000/µL [ 31 ] (Fig. 1 ). There is, therefore, a non-linear relationship between mortality and parasite densities. In a low transmission setting on the Thailand-Myanmar border, where the P. falciparum entomological inoculation rate was approximately 0.5/year, the mortality of children with > 4% P. falciparum parasitaemia (circa 200,000/µL) was 3% [ 35 , 36 ]. In that location a 3% mortality was thirty times higher than the mortality in patients with lower parasite densities, but it was five times lower than in patients who fulfilled the strict WHO definition of severe falciparum malaria [ 9 ]. As the predominant stage of parasite development determines the proportion of the parasite biomass that circulates, some patients with severe falciparum malaria have relatively low parasite densities because most of the malaria parasites are sequestered [ 32 – 34 ]. Others may have low parasite densities because they have already received anti-malarial drugs before assessment. On the other hand, a synchronous infection may have recently undergone schizogony and merozoite release resulting in a high parasite density with a predominance of young ring stage parasites. In this latter case most of the parasites in the body are circulating, and relatively few are still sequestered. Provided the patient receives an artemisinin derivative the prognosis is good. In children in areas of higher transmission, P. falciparum peripheral blood parasite densities over 200,000/uL may be tolerated with relatively few symptoms. Thus, the prognostic value of parasitaemia depends on the epidemiological setting and, overall, it is poor.
Relationship between peripheral blood parasite density and outcome in patients with acute falciparum malaria studied by Field and colleagues in Kuala Lumpur over 70 years ago [ 31 ]
Factors associated with mortality
The three main clinical presentations of severe malaria in children are coma, metabolic acidosis (usually manifest by an acidotic or “Kussmaul’s” breathing pattern, and commonly termed “respiratory distress”) and anaemia [ 2 , 10 – 13 , 22 – 26 ]. None of these are specific for malaria. These clinical presentations are major manifestations in adults too, although severe anaemia is less common. In contrast many adult patients present with acute kidney injury often accompanied by jaundice [ 37 ]. As noted earlier, there is no agreed threshold mortality threshold to define severe malaria. Among the different syndromes included in the current definition, the lowest case specific mortality is associated with malarial anaemia which can be below 1% [ 38 ]. This is still higher (by a factor of 10–100) than in uncomplicated malaria, but it is substantially lower than the mortalities associated with coma, severe metabolic acidosis, pulmonary oedema or acute renal failure (8–50%) [ 2 , 26 ]. The low mortality of severe anaemia with malaria is explained by the low sequestered parasite biomass and the inclusion, within the definition of severe malaria, of children with chronic anaemia (often as a result of repeated malaria attacks) and either incidental parasitaemia or a concomitant, otherwise uncomplicated, malaria illness. This is a very common presentation in high transmission settings where it is usually the main reason for blood transfusion in young children. The current “WHO” severe anaemia criterion requires an accompanying parasite density of 10,000/µL [ 2 ]. Densities in this range are often found in asymptomatic children, so may be incidental to the anaemia rather than causal. Even if causal the anaemia may result from a chronic process in which the parasite numbers are in a quasi-steady state, controlled by the immune response, and are very unlikely to increase further. If the parasite density requirement in the criterion for “severe anaemia” was raised it would be more specific for acute malaria but, even at higher densities, acute case specific mortalities do not rise above 5% until admission haemoglobin concentrations fall below 3 g/dL. However, it is still very important to recognize children admitted to hospital with severe malaria anaemia as a high risk group. These anaemic children have a high post-discharge mortality [ 39 – 41 ]). Furthermore they may not recover fully from their anaemia for 2–3 months after discharge. Thus, the overall mortality associated with severe malaria anaemia is significantly greater than appreciated from the acute admission [ 39 , 40 ].
The clinical syndromes
Neurological dysfunction.
The most characteristic syndrome of severe falciparum malaria is unrousable coma or cerebral malaria [ 2 , 42 ]. This diffuse, symmetrical, reversible encephalopathy may occur at any age (Fig. 2 ). The main differential diagnoses are bacterial meningoencephalitis, viral encephalitis and, in some areas, toxic encephalopathy. Cerebral malaria occurs typically in people with little or no immunity, so it is seldom seen in residents of areas of high stable malaria transmission where severe anaemia in the first years of life predominates as the manifestation of severe malaria (Fig. 3 ). The outcome of cerebral malaria depends on access to treatment and intensive care, and the degree of associated vital organ dysfunction. ‘Pure’ cerebral malaria (i.e. without other vital organ dysfunction) has approximately half the mortality of patients with coma and other organ dysfunction i.e. renal impairment, pulmonary oedema, jaundice, metabolic acidosis, or hypoglycaemia. Overall, the treated mortality of cerebral malaria in the “quinine era” was approximately 20% in adults and 12–15% in children. These mortalities have been reduced by about one third by parenteral artesunate treatment [ 17 – 20 ]. Falciparum malaria is specifically associated with convulsions, even in otherwise uncomplicated infections. The seizures are usually generalized, and they may herald the onset of coma. Although most children make a full recovery, cerebral malaria in children is associated with significant neurodevelopmental sequelae; stroke, cognitive impairment and an increased risk of epilepsy [ 42 ]. It is very important to distinguish the causal relationship between convulsions in malaria and cerebral malaria and later cognitive impairment and epilepsy, from pre-morbid conditions which may present, sometimes for the first time, as neurological dysfunction in acute malaria (and thus be misdiagnosed as cerebral malaria). Otherwise, the adverse impact of cerebral malaria on long-term neurological outcomes will be overestimated. The specificity of the diagnosis of cerebral malaria is improved by clinical and laboratory examination (see below). For example, demonstration of malaria retinopathy is highly specific for cerebral malaria as the cause of coma [ 44 ]. Severe anaemia has also been associated with neurocognitive deficits [ 45 ]. There is no evidence that severe malaria causes permanent damage to other vital organs.
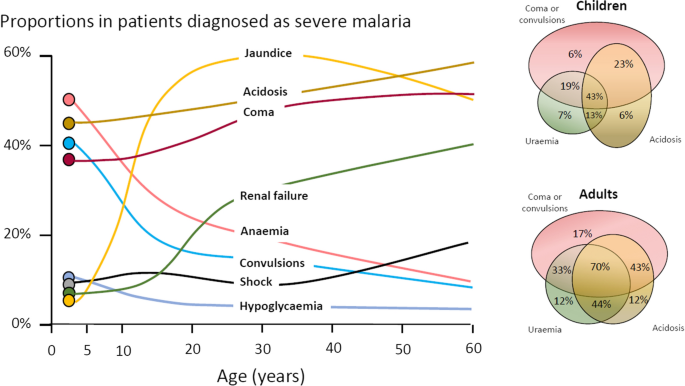
Overlap of clinical syndromes and mortalities in adults and children with severe falciparum malaria. These proportions are derived from prospective studies in SouthEast Asia and Africa of adults and children with severe falciparum malaria conducted or coordinated by the Mahidol Oxford Research Unit over the past 40 years [ 26 ]
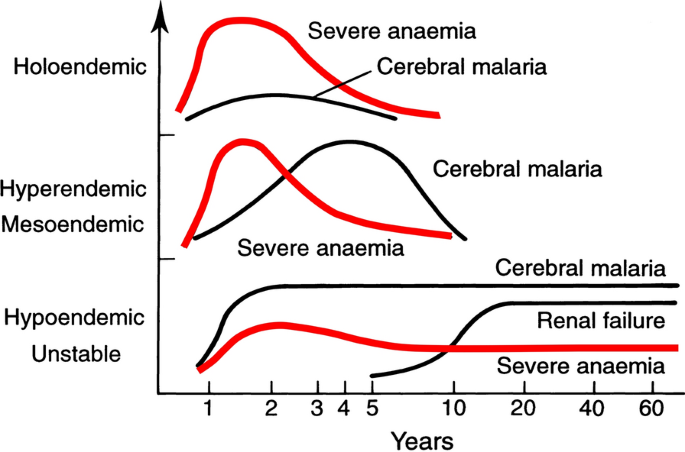
Approximate age relationships for the major clinical manifestations of severe falciparum malaria in relation to the intensity of transmission [ 53 ]. Holoendemic in this illustration approximates to a sustained entomological inoculation rate > 10 per year or a parasite rate (prevalence) in children of 0.5, and hypoendemic refers to an average entomological inoculation rate ≤ 1 year
Acidosis, kidney injury
Metabolic acidosis is a grave sign in both adults and children with severe malaria, [ 2 , 24 , 47 – 49 ] (Fig. 2 ), unless it results from very severe anaemia only, where the prognosis is better [ 38 ]. Lactate (reflecting lactic acid) accumulation is an important component of the malaria acidosis. Other organic acids, mainly of gut origin, are also significant contributors [ 46 , 50 ]. Lactic acidosis is often accompanied by hypoglycaemia reflecting anaerobic glycolysis and impaired hepatic gluconeogenesis [ 47 – 49 ]. Impaired renal function is an important manifestation of severity in younger children, but acute kidney injury (AKI) requiring renal replacement therapies is almost confined to older children and adults [ 2 , 37 , 51 ] (Figs. 2 , 3 ). The fulminant form of AKI, often associated with multiple vital organ dysfunction, is associated with a poor prognosis. In contrast the sub-acute presentation, in which plasma or serum creatinine rises steadily as the patient otherwise recovers, has a good prognosis. A period of renal replacement therapy (preferably haemofiltration or haemodialysis [ 52 ]) may be required, but there is always full recovery of renal function in survivors. The ‘hepatorenal’ combination of jaundice and renal failure became a more common presentation of severe malaria relative to cerebral malaria in Southeast Asia over the past four decades -the prognosis is worse than with AKI alone. Renal dysfunction in malaria can be misattributed in much the same way that neurological dysfunction following malaria can be overdiagnosed. In many tropical regions chronic kidney disease is common, particularly in older adults, and renal impairment may become evident for the first time during hospitalization for malaria. This may be causally attributed to malaria by mistake, and so a diagnosis of malaria nephropathy is made incorrectly. Concomitant anaemia and acidosis may also be ascribed incorrectly to malaria rather than chronic renal disease. In these misattributed cases, renal imaging, if available, often reveals small kidneys, or nephrolithiasis and hydronephrosis, and there may be biochemical or radiological evidence of metabolic bone disease.
Severe anaemia
The definitions of anaemia in malaria vary widely [ 53 ]. The most common classification—used in higher malaria transmission settings- is based on haemoglobin concentrations. In patients with acute malaria haemoglobin (Hb) concentrations between 8 g/dL and 11 g/dL are considered as mild anaemia, Hb between 5 g/dl and 8 g/dL is considered moderate, and Hb < 5 g/dL is defined as severe anaemia [ 53 ]. Unfortunately, despite their simplicity, the point of care measurements of haemoglobin concentrations, which are necessary to ensure appropriate use of blood transfusions, are often unavailable [ 54 ]. In sub-Saharan Africa the Hb ≤ 5 g/dL threshold is used widely as an indication for blood transfusion in children with malaria (whereas Hb ≤ 4 g/dL is often used for other causes of anaemia) (Fig. 4 ). The recent finding, in a large randomized trial, that children with fever (> 37.5 °C) were harmed by higher blood transfusion volumes (30 mL/kg versus 20 mL/kg) whereas children without fever benefited [ 55 – 57 ], has forced a reconsideration of blood transfusion guidelines for African children with severe anaemia [ 58 ] (Fig. 4 ). In low transmission settings an Hb ≤ 7 g/dL has been used as a transfusion indicator [ 2 ]. There is no evidence to support this threshold. Anaemia is the main severe manifestation of malaria in areas of high transmission, where it is largely confined to young children [ 59 ] (Fig. 2 ). Severe anaemia, as a criterion of severe malaria, encompasses a spectrum of aetiologies with several different, but often overlapping, pathological processes which are still not well understood [ 53 ]. At one end of the disease spectrum is an acute illness in patients with high parasite biomass infections and rapid destruction of parasitized and unparasitized red cells. The unparasitized cells comprise the majority of erythrocytes lost. Haemolysis is sometimes sufficient to result in haemoglobinuria (blackwater fever). However, malaria is not the only cause of blackwater fever, which, after over 120 years of investigation, still remains a puzzle [ 60 – 64 ]. Massive haemolysis may occur in any epidemiological setting. At the other end of the disease spectrum, in settings of high transmission or poor access to treatment, are patients (usually young children) with chronic anaemia and incidental parasitaemia. Repeated or untreated malaria infections resulting in shortened erythrocyte survival and protracted dyserythropoeisis are important contributors to this chronic, or acute on chronic, syndrome [ 52 ].
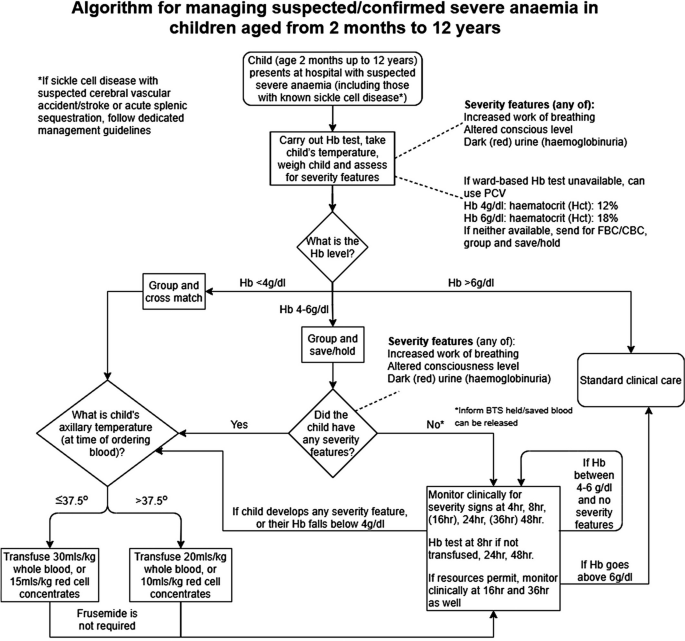
Proposed algorithm for managing suspected/confirmed severe anaemia in African children aged from 2 months to 12 years [ 58 ]
Improved malaria control reduces the frequency of malaria infections and thus the prevalence of severe anaemia [ 59 , 65 ]. As in chronic inflammatory conditions, malaria is associated with iron deficiency [ 66 ]. Other common causes of anaemia in malaria endemic regions are nutritional deficiencies, hookworm, bacterial infections and haemoglobinopathies. Bacterial infections are also associated with acute anaemia presentations [ 67 ]. At presentation to hospital the short-term prognosis of severe anaemia is relatively good as the anaemia is mainly chronic and partially compensated (by the right shifted oxygen dissociation curve). If blood transfusion can be given promptly then the acute mortality is low but, in higher malaria transmission settings, hospitalization for severe anaemia identifies children who are at increased risk of subsequent death. Approximately 5% will die within 6 months. Post-discharge anti-malarial chemoprophylaxis provides temporary protection, which suggests that recurrent malaria is a major contributor to this high mortality [ 40 , 41 ]. The prognosis of children hospitalized with severe anaemia is much better than for the other severe manifestations of falciparum malaria but, because of the longer-term impact, and because it is so common in high transmission settings, the adverse impact at a population level is substantial [ 59 ]. Deaths from malaria overall are positively correlated with transmission intensity [ 59 ], and the direct or indirect consequences of severe anaemia are major contributors to this relationship.
Other complications
Pulmonary oedema (ARDS) carries a very high mortality in falciparum malaria- even with positive pressure ventilation. It often occurs after the other severe manifestations have become evident. Pulmonary oedema results from increased pulmonary capillary permeability. Pulmonary oedema may also occur in vivax malaria, where the prognosis is much better [ 2 ]. Liver dysfunction is usual in severe malaria [ 68 ] although liver failure, as in viral or toxic hepatic injury, never occurs [ 2 ]. Profound thrombocytopenia is associated with an increased mortality in severe malaria, but it is not an independent risk factor and, contrary to some reports, it is not regarded as a criterion of severe malaria [ 69 ]. Although thrombocytopenia is usual in all malarias and coagulation indices are often abnormal in severe illness, significant bleeding (if present, usually from the stomach) and clinically significant coagulopathy are unusual in severe malaria. Overall, the probability of death from severe falciparum malaria depends on the extent and degree of vital organ dysfunction and the access to appropriate treatment [ 2 , 70 ]. Secondary bacterial infection is a potentially lethal complication, particularly in African children. Approximately 6% of children diagnosed with severe malaria have concomitant bacteraemia [ 71 ]. In adults the incidence is much lower (1%) [ 72 ]. Misdiagnosis (see below) is common [ 73 ], as it is difficult to differentiate between severe malaria with concomitant bacteraemia and a primary bacterial infection with incidental parasitaemia [ 74 , 75 ].
Pathophysiology of severe falciparum malaria
Similar to some primate malaria parasites ( P. fragile, P. coatneyi ), but unlike the other human malaria parasites, P. falciparum causes the infected erythrocyte to cytoadhere to vascular endothelium after the first third of the asexual blood cycle [ 2 ]. Severe falciparum malaria results from the extensive sequestration of erythrocytes containing these mature parasite forms in the microvasculature of vital organs [ 2 , 3 , 76 , 77 ] (Fig. 5 ). The microvascular obstruction by highly metabolically active cells, consequent cellular dysfunction, and the liberation of large quantities of bioactive haem are considered the main pathological processes in severe falciparum malaria [ 70 , 76 – 79 ]. There are secondary consequences on vascular function, permeability, tone and on cellular transport. Thus, vital organ dysfunction depends on the extent and the location of parasitized erythrocyte sequestration. The extent of sequestration is heterogeneous, even at a microvascular level [ 80 ]. Magnetic resonance cerebral imaging in paediatric cerebral malaria shows a variety of different patterns. The brain is usually swollen, with restricted diffusion and variable evidence of oedema [ 81 ]. Isolated restricted white matter diffusion is associated with a better prognosis, while oedema is associated with a worse prognosis and an increased risk of sequelae [ 82 , 83 ]. The sequestered static red blood cells occupy space and cause cerebral engorgement [ 3 , 4 ] which contributes to raised intracranial pressure. Cytoadherent parasitised erythrocytes are not the only contributors to disease severity. Very high parasitaemias caused by non-sequestering malaria parasites cause severe malaria across the animal kingdom, and the simian parasite P. knowlesi is potentially lethal in humans – but these parasites do not cause cerebral malaria [ 2 , 84 ]. At very high parasite densities, erythrocyte dysfunction contributes to aggregation and impaired microcirculatory flow and oxygen delivery without cytoadherence. The precise causes of acute kidney injury and acute pulmonary oedema in severe malaria are unclear. Despite extensive research and much speculation over many years, there is little evidence for a primary immunopathological process in severe malaria, or for a final common pathological pathway with bacterial sepsis involving pro-inflammatory cytokine release. As described earlier, the pathobiology of severe malaria has been rich ground for hypothesis and speculation, often fueled by observations in a murine model, which is readily studied in the laboratory but has very little similarity to the human disease [ 5 ]. Observations in the murine ‘model’ have led to a long list of putative adjuvant interventions -all of which have proved either ineffective or harmful. This emphasizes the importance of distinguishing causal pathological processes in malaria from their consequences. From a clinical and operational perspective, it is essential to distinguish causal processes in severe malaria [ 70 ] from those processes in other severe infections with which severe malaria is very often confused (notably bacterial infections). The implications of misdiagnosis on operational disease management and pathobiology understanding are discussed below.
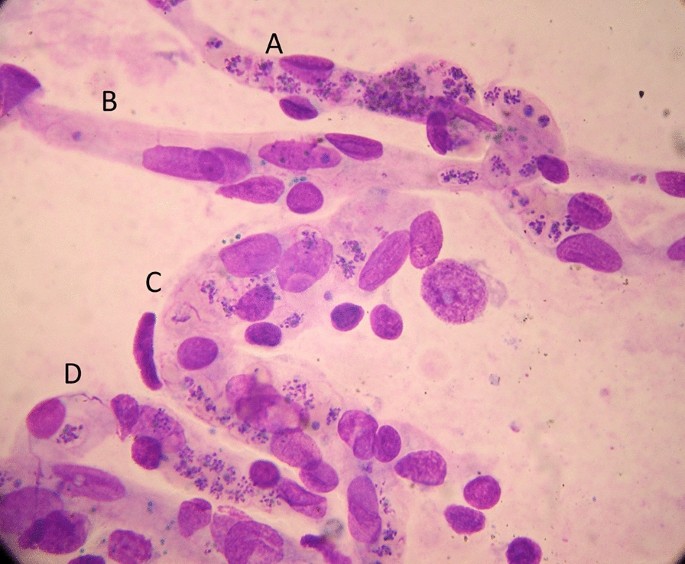
Brain smear from fatal cerebral malaria. The vessels ( A , C and D ) are packed with red cells containing P. falciparum schizonts (many of which are disrupted) and malaria pigment (haemozoin). Vessel segment B , by contrast, contains mainly unparasitized erythrocytes
The diagnosis of severe falciparum malaria
Severe malaria is a medical emergency. Appropriate immediate management is life-saving. An initial brief clinical examination assessing vital signs, peripheral perfusion, respiratory pattern, anaemia, jaundice and level of consciousness, and confirming the absence of rash should be followed rapidly by a blood smear or RDT confirmation [ 2 ]. In a low transmission setting, or with imported malaria, the diagnosis is straightforward. The results of a thin blood film or RDT can be available within minutes of taking a blood sample. Treatment should not be delayed if the blood results take longer than this. Microscopy examination of thin and thick blood smears provides both diagnostic and prognostic information; the parasite count, the parasite stage of development and the presence of neutrophil ingested pigment all have prognostic value and are readily assessed [ 2 , 32 , 33 , 85 – 87 ]. If the parasitaemia is high, the thin film assessment can take less than one minute. The RDT does not provide this quantitative prognostic information. In addition, the Pf HRP2 based RDTs can remain positive for days or weeks following a previous infection [ 88 ]. On the other hand, RDTs are useful in excluding a mixed P. falciparum infection in a patient with a blood slide diagnosis of vivax, malariae or ovale malaria [ 89 ], and they provide a diagnosis in patients who have received treatment with artemisinins several days previously and who are still severely ill (but have cleared their parasitaemia). This is common in adults presenting with acute kidney injury, which may take days or weeks to recover fully. In a low transmission setting, finding malaria parasites in the peripheral blood (by microscopy or RDT) is highly specific for malaria as the cause of illness. PCR diagnosis and speciation has proved very valuable in epidemiological studies, but PCR has no role in the acute diagnosis of severe malaria in endemic areas. It is too slow to be reported and it is too sensitive. PCR detects a higher proportion of people with previously asymptomatic (i.e. incidental) parasitaemia and therefore results in even more misdiagnosis of severe malaria.
At higher levels of transmission, the diagnosis of malaria as the cause of the presenting illness is much more difficult. The prevalence of microscopy or RDT detectable parasitaemia in apparently healthy individuals increases with transmission intensity, so the possibility of ascribing malaria incorrectly as the cause of illness rises too [ 90 ]. In sub-Saharan Africa a high proportion of apparently healthy children have detectable malaria parasitaemia. So how can severe illness caused by malaria parasites be distinguished from severe illness caused by something else with coincident parasitaemia? Good clinical examination is important but diagnostic uncertainty often persists. Other sites and sources of infection should be sought. In unconscious patients a lumbar puncture should be performed to exclude bacterial meningoencephalitis. Sequestration can be seen in-vivo by skilled indirect ophthalmoscopy along with other changes termed “malaria retinopathy” which have high specificity for cerebral malaria [ 44 , 92 – 94 ]. The buccal or rectal microcirculations can be visualized by direct orthogonal polarized light imaging [ 76 , 95 ]. In fatal cases sequestration can be demonstrated in the capillaries and venules of the brain in a post-mortem needle biopsy [ 80 , 96 , 97 ] (Fig. 5 ). But none of these specialist techniques are available in most places where severe malaria is managed. However, most hospitals and many health centres do have microscopes, and many centres now can perform full blood counts. Brief microscopy examination of a stained thin blood film provides valuable diagnostic and prognostic information [ 85 – 87 ]. The blood count is also informative (see below). Point of care blood glucose and lactate measurement is very important, particularly in unconscious or obtunded patients.
The immediate management of severe malaria
The outcomes of severe malaria and of severe sepsis are critically dependent on rapid access to health care and immediate treatment. Delays in giving artesunate and antibiotics are potentially lethal. Sadly, additional delays may still occur after the patient has reached hospital. Any patient suspected of having severe malaria should be treated as such [ 2 ].
Pre-referral
Severe malaria often presents initially far from the health centre or hospital. Referral for medical care can take hours, or sometimes days. At the community level, where giving parenteral drugs is not possible, pre-referral treatment of severe malaria with rectal artesunate reduces mortality by about 25% [ 98 ]. This community-based intervention has been very slow to be deployed, and now the WHO has recommended that it be stopped [ 99 ]. This recent WHO moratorium followed preliminary analysis of a large sequential observational study (“CARAMAL”) in Nigeria, Uganda and the Democratic Republic of the Congo [ 100 ]. Mortality reportedly increased after rectal artesunate was deployed, attributed to delays in the referral of severely ill children. However, there are serious concerns over the design of the study, potential major confounders, the accuracy of the diagnosis, and particularly—the causal interpretation of the results [ 101 ]. The CARAMAL study identified important problems with the referral of severely ill children, but it should not be used to evaluate the effectiveness of pre-referral rectal artesunate. The WHO moratorium appears to be a mistake. Rectal artesunate should be deployed to counter lethal delays in the referral of severe malaria. There are no pre-referral rectal antibiotic formulations unfortunately.
Health centre or hospital
At the level of the health centre or hospital in an area of higher malaria transmission (i.e. most of sub-Saharan Africa), the difficulty in distinguishing malaria from sepsis in children means that both parenteral anti-malarials (i.e. artesunate 3 mg/kg stat for children < 20 kg and 2.4 mg/kg for larger patients) and broad-spectrum antibiotics should be given together as soon as the diagnosis is suspected [ 2 , 73 ]. The most widely used empirical antibiotic treatment of severe sepsis is parenteral ceftriaxone. Administration of antibiotics should not be delayed. The drugs are very safe. Giving anti-malarials initially does no harm if the infection turns out to be bacterial or viral, and giving antibiotics does no harm if the infection is severe malaria only. Immediate administration of parenteral artesunate and broad-spectrum antibiotics to a child suspected of having severe malaria is the single most important life-saving intervention .
In low transmission settings where misdiagnosis is much less likely, it is reasonable in adults to treat only for severe malaria unless there is evidence for concomitant bacterial sepsis. However, antibiotics should be given to all adult patients with a very high parasitaemia (> 20%) [ 72 ], and should be given immediately if there is any unexplained clinical deterioration.
The misdiagnosis of severe malaria
Misdiagnosis of severe malaria is common. Its impact is underestimated. Misdiagnosis can result in incorrect treatment [ 73 ] and it dilutes and distorts genetic, epidemiology, burden of disease, long term impact, pathophysiology and therapeutic studies. In areas of higher transmission (e.g. Sub-Saharan Africa, Oceania), children are often diagnosed as having severe malaria because the blood test is “positive” but, in fact, they have another infection (often bacterial sepsis) causing their severe illness [ 90 ]. As severe bacterial infections have a higher mortality than severe malaria, and require antibiotic treatment, it is essential that both are treated immediately.
The relationship between malaria and bacterial infections is complex [ 71 – 75 , 102 – 109 ]. Severe malaria predisposes to bacterial infections. In a large prospective series of Vietnamese adults with strictly defined severe falciparum malaria (in whom diagnostic specificity for severe malaria is very high), the overall incidence of concomitant septicaemia (identified by positive blood culture) was 1.1% [ 72 ]. Hyperparasitaemia was a risk factor for bacteraemia; in patients with > 20% parasitemia the prevalence of concomitant bacteremia was 5.2%, whereas it was eight times lower (0.65%) in patients with lower parasitaemias. Concomitant bacteraemia is much more frequent in African children diagnosed with severe malaria. Approximately 6% of children hospitalized with a diagnosis of severe falciparum malaria in Africa are also bacteraemic [ 71 ]. As blood cultures are insensitive (but more specific- at least for most organisms) in diagnosis, the true proportion is likely to be much higher. Recent probabilistic assessments based on platelet and white blood cell counts, and also a quantitative parasite biomass indicator (plasma P f HRP2) [ 110 , 111 ] measured in large prospective studies of severe malaria in children, suggest that approximately one third of children diagnosed as having severe malaria in leading research centres actually had another condition (likely mainly sepsis) as the main cause of their illness [ 108 , 109 ] (Fig. 6 ). These probabilistic assessments were validated by comparing the prevalences of sickle cell trait (HbAS), which provides strong protection against severe malaria, between the two groups. The prevalence of HbAS was substantially lower in children with ‘true’ severe malaria than it was in those with a different cause of severe illness. Even for the relatively specific syndrome diagnosed as cerebral malaria, a post-mortem examination study, conducted in a leading research centre in Malawi, revealed a different pathology in one quarter of cases [ 112 ].
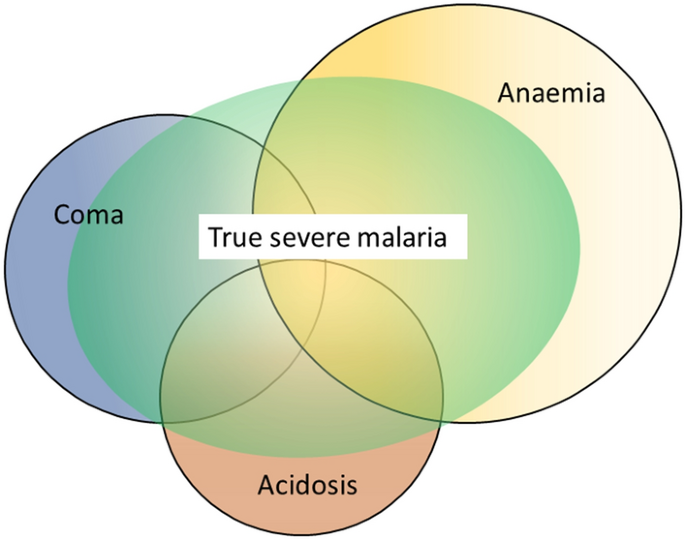
Misdiagnosis of severe falciparum malaria in African children -approximate relationships. [ 108 , 109 ]
The substantial overdiagnosis of severe malaria cannot be ignored in epidemiology, burden of disease, pathophysiology, genetic association and treatment studies. In the large evaluation of African children who had been admitted to leading research centres with a diagnosis of severe malaria (described above), mortality was higher in the likely misdiagnosed group, presumably because most had sepsis [ 108 , 109 ]. This suggests that malaria attributable mortality in African children may have been overestimated. If it has indeed been overestimated then the benefits of the substantial investments in malaria control measures and the provision of effective drugs (i.e. ACTs) have been underestimated [ 113 ]. Progress in reducing the number of deaths from severe malaria may have been better than estimated currently. The high rates of misdiagnosis, even in expert research centres, should be also accomodated by those formulating treatment guidelines and policies for severe malaria. Prompt, or preferably pre-referral, antibiotics must be given together with artesunate. Misdiagnosis also probably explains the difference in mortality reduction with artesunate compared with quinine in adults and children in Asia (where diagnostic specificity is high) compared with children in Africa (where diagnostic specificity is lower) (22.5%) [ 5 , 18 , 19 ]. In Asia the mortality reduction was 35% compared with 22.5% in African children (Fig. 7 ).
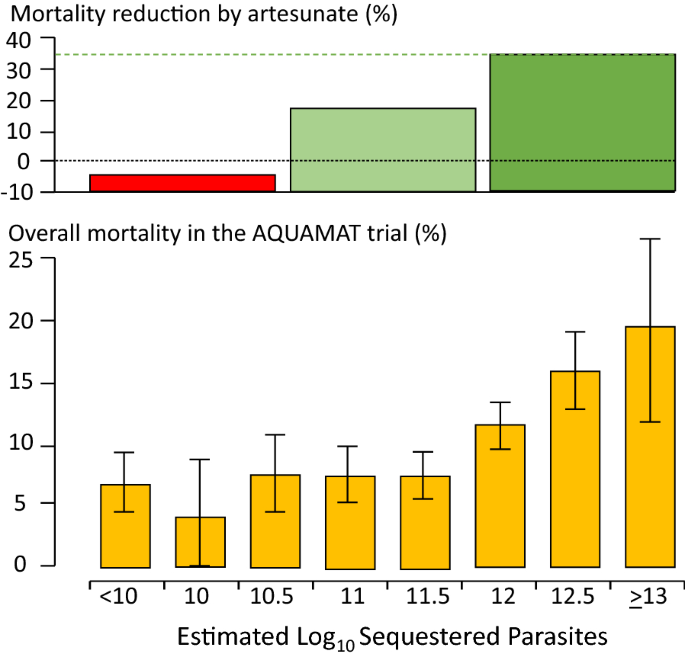
Relationship between estimated parasite biomass and mortality [ 4 , 110 ] in the large randomized controlled trial which compared artesunate and quinine in African children with severe malaria (AQUAMAT) [ 19 ]. The upper panel divides the patients into tertiles by treatment effect (reduction in mortality by artesunate). The mortality reduction in the preceding randomized controlled trial (SEAQUAMAT) which compared artesunate and quinine in Southeast Asia (where the diagnosis of severe malaria is more specific) is shown for comparison [ 18 ] (upper green dashed line). There was no treatment benefit from artesunate in patients in the lowest tertile of parasite biomass (red), likely corresponding to patients with another cause of severe illness (probably sepsis) and incidental parasitaemia [ 108 , 109 ]. The lower panel shows the corresponding relationship between mortality in the AQUAMAT study and the estimated total parasite numbers in the body derived from the admission plasma Pf HRP2 concentration [ 110 ]
Thus, it seems that some of the children with bacteraemia who are diagnosed as having severe malaria may genuinely have a high parasite biomass and extensive sequestration predisposing to bacterial sepsis—but the remainder have a primary bacterial infection and incidental or concomitant malaria. The interaction is complicated further as severe malarial anaemia predisposes to bacterial sepsis, and patients with uncomplicated malaria may have concomitant sepsis. At a population level, as malaria is controlled, the prevalence of sepsis declines (and so does the apparent protective benefit of HbAS against bacterial infections) pointing to the important contribution of malaria to bacterial sepsis, both concomitantly and sequentially [ 104 ]. It is very likely that the same problem of misdiagnosis occurs with Plasmodium vivax. In endemic areas low density chronic P. vivax parasitaemia is common, and so it is not unusual for severely ill patients to have incidental low-density infections, particularly if PCR is used for parasite detection.
The consequences of severe malaria
Children who are admitted with severe malaria anaemia have a high mortality in the months following admission [ 39 – 41 ]. This can be reduced by giving effective antimalarial prophylaxis, which indicates that repeated malaria infection is associated with death. Seizures and coma are associated with neurological deficit in surviving children [ 42 , 114 ]. The deficit is evident immediately following recovery in approximately 10% of children following cerebral malaria [ 115 ]. In two thirds of these cases the clinical picture is of stroke (suggesting a large cerebral vessel territory has been compromised). While many children recover fully, other deficits and behavioural and mental health problems often become apparent -particularly with detailed psychomotor and behavioural evaluation [ 42 , 114 , 116 – 118 ]. Epilepsy is increasingly recognized. These later onset epileptic, psychomotor and behavioural abnormalities may result from cerebral malaria, but they may also be pre-morbid conditions revealed by acute malaria [ 44 ].
Implications for the assessment and treatment of patients diagnosed with severe falciparum malaria
Overall, the consensus definitions of severe malaria described generally as “WHO criteria” have worked well to identify patients at risk and to inform research studies. From a practical case management perspective, specificity in the diagnosis is not as important as recognition that severe malaria could be the cause of the severe illness, and thus starting life-saving treatment with artesunate as soon as possible [ 2 ]. A new simple to administer artesunate formulation is under development. In children with suspected severe malaria in higher transmission settings parenteral broad-spectrum antibiotics should also be given immediately in all cases. As delay in receiving artesunate is a major contributor to death, it is important that referral to a facility capable of managing the sick patient should be as rapid as possible. Pre-referral rectal artesunate should be given to all children with suspected severe malaria [ 2 , 98 ]. The WHO moratorium [ 99 ] on rectal artesunate will hopefully soon be lifted [ 101 ]. Pre-referral antibiotic formulations should be developed.
For patients needing respiratory support, artificial ventilation has improved in recent years as the dangers of high inflation pressures have become evident [ 119 ]. Unfortunately, ventilators and trained staff are often unavailable in the areas where severe malaria is common. Otherwise, apart from the replacement of quinine by artesunate, the overall recommended management of severe malaria has changed relatively little over the past few decades. Aggressive fluid management (as in sepsis) [ 76 , 120 ], high volume (30 mL/kg) blood transfusions (in febrile children)[ 56 ], mannitol to reduce brain swelling [ 121 , 122 ], and unproven adjuvant therapies [ 5 ] have all proved harmful. Studies to optimize blood transfusion and fluid management are ongoing, but the general consensus is returning back to more cautious fluid management in severe malaria [ 7 , 123 ]. Evidence to date does not support red cell concentrates over whole blood in immediate management [ 57 ]. The optimum prevention and treatment of convulsions still remains uncertain. In a large randomized trial, conducted in a centre without access to artificial ventilation, seizure prevention by full dose prophylactic phenobarbitone increased mortality because of respiratory depression [ 16 ]. In a small trial levetiracetam proved safer [ 124 ], and may well become the anticonvulsant of choice, as it is in other settings, although more evidence is needed. Fosphenytoin was ineffective [ 125 ]. Renal replacement should start early in adults, blood glucose should be tested frequently and hypoglycaemia treated promptly [ 2 ]. Studies are ongoing to determine if paracetamol could attenuate renal injury in severe malaria [ 51 ]. If broad spectrum antibiotics have not been started (e.g. in adults in low transmission settings) there should be a low threshold for giving them if the patient deteriorates [ 2 ] -particularly in hyperparasitaemic patients [ 72 ].
From a research or epidemiology perspective, the low specificity of the current definition of severe malaria in African children is a challenge (Fig. 6 ). It has diluted therapeutic evaluations and distorted pathophysiology interpretations and genetic association studies. Most of the techniques to improve the specificity of diagnosis (notably indirect ophthalmoscopy or other methods of visualizing the microcirculation, or measurement of parasite biomass indicators such as plasma Pf HRP2 (Fig. 7 ) or plasma Pf DNA concentrations) are not readily available [ 91 – 95 , 110 , 111 , 126 ]—although simple dilution of a plasma sample and testing (by eye) with a Pf HRP2 RDT is not too difficult [ 127 ]. Importantly, the time-honoured peripheral thin blood smear does contain valuable information. Sadly, it is underused as a diagnostic and as a prognostic tool, and in many centres has been supplanted by the malaria rapid test, which, as currently used, does not provide prognostic information. In blood slides with parasitaemias over 0.5% the stage of parasite development can be easily and rapidly evaluated by microscopy. For any parasite density, finding > 50% tiny rings carries a relatively good prognosis whereas if > 20% parasites contain visible malaria pigment the prognosis is worse [ 85 ]. The proportion of neutrophils containing malaria pigment is also a very useful and readily assessed both for diagnosis and for prognostic assessment [ 86 , 87 ]. Most health facilities have at least one microscope – but sadly it is often old, fungus infested and accompanied by dirty slides, waterlogged methanol and outdated unfiltered stains. Malaria microscopy is well established but it is not well supported, and it is not prioritized in current malaria control funding. Hospital and health centres managing severe malaria should support good microscopy as an essential diagnostic and prognostic measure. Blood counts are valuable too. The haemoglobin concentration or haematocrit guides blood transfusion. The differential white count provides diagnostic information. Although severe malaria may be accompanied by leukocytosis, finding a high neutrophil count (often with toxic granules) together with lymphopenia points to bacterial sepsis. Thrombocytopenia is usual in severe malaria, but not in sepsis. In the recent large probabilistic assessments of severe malaria in African children, the combination of a platelet count of ≤ 150,000/μl and a plasma Pf HRP2 concentration of ≥ 1000 ng/ml had an estimated sensitivity of 74% and specificity of 93% in identifying true severe falciparum malaria [ 109 ] (Table 3 ). Future studies of severe malaria should always include differential blood counts, platelet counts and, preferably, a parasite biomass indicator. The anaemia criterion to define severe malaria should be reviewed.

Severe malaria caused by other malaria species
Plasmodium knowlesi, with its quotidian cycle, can sometimes cause fulminant infections in humans [ 84 , 128 , 129 ]. It does not sequester markedly so the parasite count is a good guide to biomass. P. knowlesi infections do not cause coma (cerebral malaria) but they can cause the other potentially lethal manifestations of severe malaria. Morphologically the younger P. knowlesi parasites resemble P. falciparum , whereas the older forms are often mistaken for Plasmodium malariae . Indeed any P. malariae parasitaemia over 1% should be regarded as P. knowlesi until proved otherwise. Uncomplicated P. vivax infections in a non-immune subject are often worse than uncomplicated P. falciparum malaria infections, causing high fever, weakness, malaise and sometimes rigors and prostration. Some of these vivax malaria illnesses warrant hospital admission. In the past 20 years there has been a marked increase in the number of reports of “severe” vivax malaria, mainly from India [ 130 – 132 ]. In some of the reports, the basis for the classification has been thrombocytopenia, which is not generally regarded as a criterion for severe malaria. Some patients hospitalized with P. vivax malaria die, particularly if they are old or debilitated [ 133 , 134 ]. P. vivax may sometimes cause acute pulmonary oedema-although the prognosis is better than in severe falciparum malaria [ 133 , 135 ]. But severe vivax malaria is overdiagnosed for the same reasons that severe falciparum malaria is overdiagnosed. Incidental parasitaemias are found in patients with severe anaemia or vital organ dysfunction and a causal relationship is inferred. In low transmission settings (i.e. most P. vivax endemic areas) Plasmodium vivax can cause severe illness, but the proportion of symptomatic cases which develop life-threatening illness is substantially less than for P. falciparum infections. However recurrent infections with P. vivax in areas of high transmission, such as the island of New Guinea, are associated with severe anaemia and substantial mortality both in the acute phase and over the longer term [ 136 – 138 ]. Further large and detailed cohort studies of hospitalized P. vivax infections would help clarify the prognostic associations and risk factors. But overall, the mortality of acute P. vivax infections is substantially lower than that of P. falciparum infections.
Conclusions
The apparent lack of progress in reducing the global death toll from malaria despite substantial investment suggests that we should reexamine the evidence, and review the current strategies to prevent and treat severe malaria [ 113 ]. The mortality of this common but frequently misdiagnosed syndrome can and should be reduced. Severe malaria deserves more attention.
Availability of data and materials
Review—individual trial data from trials conducted by MORU can be requested from the MORU data access committee.
Torti F. Therapeutice specialis ad febres quasdam perniciosas, (Venice 1712)
World Health Organization. Severe Malaria. Trop Med Int Health. 2014;19(Suppl 1):1–131.
Google Scholar
Marchiafava E, Bignami A. On summer-autumn malarial fevers. In: Marchiafava E, editor. Two monographs on malaria and the parasites of malarial fevers (translated from the first Italian edition by JH Thompson). London: New Sydenham Society; 1894. p. 1–232.
White NJ, Turner GD, Dondorp AM, Day NP. Lethal malaria: Marchiafava and Bignami were right. J Infect Dis. 2013;208:192–8.
Article CAS PubMed PubMed Central Google Scholar
White NJ, Turner GD, Medana IM, Dondorp AM, Day NP. The murine cerebral malaria phenomenon. Trends Parasitol. 2010;26:11–5.
Article PubMed PubMed Central Google Scholar
Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet. 1976;1:1031–4.
Article CAS PubMed Google Scholar
World Health Organization Malaria Action Programme. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1986;80(suppl):1–50.
Warrell DA, Looareesuwan S, Warrell MJ, Kasemsarn P, Intaraprasert R, Bunnag D, et al. Dexamethasone proves deleterious in cerebral malaria A double-blind trial in 100 comatose patients. N Engl J Med. 1982;306:313–9.
World Health Organization Division of Control of Tropical Diseases. Severe and complicated malaria. 2nd edn. Trans R Soc Trop Med Hyg 1990;2:1‑65.
Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Quart J Med. 1989;71:441–59.
CAS PubMed Google Scholar
Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404.
Waller D, Krishna S, Crawley J, Miller K, Nosten F, Chapman D, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21:577–87.
World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1-90.
Article Google Scholar
Tran TH, Day NP, Nguyen HP, Nguyen TH, Tran TH, Pham PL, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335:76–83.
van Hensbroek MB, Palmer A, Onyiorah E, Schneider G, Jaffar S, Dolan G, et al. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J Infect Dis. 1996;174:1091–7.
Article PubMed Google Scholar
Crawley J, Waruiru C, Mithwani S, Mwangi I, Watkins W, Ouma D, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet. 2000;355:701–6.
The Artemether-Quinine Meta-analysis Study Group. A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:637–50.
Dondorp A, Nosten F, Stepniewska K, Day N, White NJ; South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005; 366: 717–25.
Dondorp AM, Fanello CE, Hendriksen ICE, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–57.
Phu NH, Tuan PQ, Day N, Mai NT, Chau TT, Chuong LV, et al. Randomized controlled trial of artesunate or artemether in Vietnamese adults with severe falciparum malaria. Malar J. 2010;9:97.
Kremsner PG, Taylor T, Issifou S, Kombila M, Chimalizeni Y, Kawaza K, et al. A simplified intravenous artesunate regimen for severe malaria. J Infect Dis. 2012;205:312–9.
Taylor T, Olola C, Valim C, Agbenyega T, Kremsner P, Krishna S, et al. Standardized data collection for multi-center clinical studies of severe malaria in African children: establishing the SMAC network. Trans R Soc Trop Med Hyg. 2006;100:615–22.
Idro R, Aloyo J, Mayende L, Bitarakwate E, John CC, Kivumbi GW. Severe malaria in children in areas with low, moderate and high transmission intensity in Uganda. Trop Med Int Health. 2006;11:115–24.
English M, Waruiru C, Amukoye E, Murphy S, Crawley J, Mwangi I, et al. Deep breathing in children with severe malaria: indicator of metabolic acidosis and poor outcome. Am J Trop Med Hyg. 1996;55:521–4.
Imbert P, Sartelet I, Rogier C, Ka S, Baujat G, Candito D. Severe malaria among children in a low seasonal transmission area, Dakar, Senegal: influence of age on clinical presentation. Trans R Soc Trop Med Hyg. 1997;91:22–4.
von Seidlein L, Olaosebikan R, Hendriksen IC, Lee SJ, Adedoyin OT, Agbenyega T, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54:1080–90.
WHO. Guidelines for the treatment of malaria. Geneva, World Health Organization, 2015.
Swellengrebel NH, de Buck A. Malaria in The Netherlands: Scheltema & Holkema Ltd. Amsterdam, 1938.
Snounou G, Pérignon JL. Malariotherapy–insanity at the service of malariology. Adv Parasitol. 2013;81:223–55.
Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis. 2011;11:541–56.
Field JW. Blood examination and prognosis in acute falciparum malaria. Trans R Soc Trop Med Hyg. 1949;43:33–48.
White NJ, Chapman D, Watt G. The effects of multiplication and synchronicity on the vascular distribution of parasites in falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:590–7.
Kingston HW, Ghose A, Plewes K, Ishioka H, Leopold SJ, Maude RJ, et al. Disease severity and effective parasite multiplication rate in falciparum malaria. Open Forum Infect Dis. 2017;4:169.
Intharabut B, Kingston HW, Srinamon K, Ashley EA, Imwong M, Dhorda M, et al. Artemisinin resistance and stage dependency of parasite clearance in falciparum malaria. J Infect Dis. 2019;219:1483–9.
Luxemburger C, Thwai KL, White NJ, Webster HK, Kyle DE, Maelankirri L, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90:105–11.
Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997;91:256–62.
Trang TT, Phu NH, Vinh H, Hien TT, Cuong BM, Chau TTH, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15:874–80.
Brand NR, Opoka RO, Hamre KE, John CC. Differing causes of lactic acidosis and deep breathing in cerebral malaria and severe malarial anemia may explain differences in acidosis-related mortality. PLoS ONE. 2016;11: e0163728.
Phiri KS, Calis JC, Faragher B, Nkhoma E, Ng’oma K, Mangochi B, et al. Long term outcome of severe anaemia in Malawian children. PLoS ONE. 2008;3: e2903.
Kwambai TK, Dhabangi A, Idro R, Opoka R, Watson V, Kariuki S, et al. Malaria chemoprevention in the post-discharge management of severe anemia. N Engl J Med. 2020;383:2242–54.
Kwambai TK, Mori AT, Nevitt S, van Eijk AM, Samuels AM, Robberstad B, et al. Post-discharge morbidity and mortality in children admitted with severe anaemia and other health conditions in malaria-endemic settings in Africa: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2022;6:474–83.
Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–40.
Wattanagoon Y, Srivilairit S, Looareesuwan S, White NJ. Convulsions in childhood malaria. Trans R Soc Trop Med Hyg. 1994;88:426–8.
Birbeck GL, Beare N, Lewallen S, Glover SJ, Molyneux ME, Kaplan PW, et al. Identification of malaria retinopathy improves the specificity of the clinical diagnosis of cerebral malaria: findings from a prospective cohort study. Am J Trop Med Hyg. 2010;82:231–4.
Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, Romero RA, et al. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis. 2014;59:336–44.
Herdman MT, Sriboonvorakul N, Leopold SJ, Douthwaite S, Mohanty S, Hassan MM, et al. The role of previously unmeasured organic acids in the pathogenesis of severe malaria. Crit Care. 2015;19: e317.
White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, et al. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–6.
White NJ, Miller KD, Marsh K, Berry CD, Turner RC, Williamson DH, et al. Hypoglycaemia in African children with severe malaria. Lancet. 1987;11:708–11.
Taylor TE, Molyneux ME, Wirima JJ, Fletcher KA, Morris K. Blood glucose levels in Malawian children before and during the administration of intravenous quinine for severe falciparum malaria. N Engl J Med. 1988;319:1040–7.
Leopold SJ, Ghose A, Allman EL, Kingston HWF, Hossain A, Dutta AK, et al. Identifying the Components of Acidosis in Patients With Severe Plasmodium falciparum Malaria Using Metabolomics. J Infect Dis. 2019;219:1766–76.
Plewes K, Turner GDH, Dondorp AM. Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr Opin Infect Dis. 2018;31:69–77.
Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347:895–902.
White NJ. Anaemia and malaria. Malar J. 2018;17: e371.
Uyoga S, George EC, Bates I, Olupot-Olupot P, Chimalizeni Y, Molyneux EM, et al. Point-of-care haemoglobin testing in African hospitals: a neglected essential diagnostic test. Br J Haematol. 2021;193:894–901.
Maitland K, Kiguli S, Olupot-Olupot P, Engoru C, Mallewa M, Saramago Goncalves P, et al. Immediate Transfusion in African Children with Uncomplicated Severe Anemia. N Engl J Med. 2019;381:407–19.
Maitland K, Olupot-Olupot P, Kiguli S, Chagaluka G, Alaroker F, Opoka RO, et al. Transfusion volume for children with severe anemia in Africa. N Engl J Med. 2019;381:420–31.
George EC, Uyoga S, M'baya B, Kyeyune Byabazair D, Kiguli S, Olupot-Olupot P et al. Whole blood versus red cell concentrates for children with severe anaemia: a secondary analysis of the transfusion and treatment of African children (TRACT) trial. Lancet Glob Health. 2022;10:e360-e368
Maitland K, Kiguli S, Olupot-Olupot P, Opoka RO, Chimalizeni Y, Alaroker F, et al. Transfusion management of severe anaemia in African children: a consensus algorithm. Br J Haematol. 2021;193:1247–59.
Paton RS, Kamau A, Akech S, Agweyu A, Ogero M, Mwandawiro C, et al. Malaria infection and severe disease risks in Africa. Science. 2021;373:926–31.
Tran TH, Day NP, Ly VC, Nguyen TH, Pham PL, Nguyen HP, et al. Blackwater fever in southern Vietnam: a prospective descriptive study of 50 cases. Clin Infect Dis. 1996;23:1274–81.
Bodi JM, Nsibu CN, Longenge RL, Aloni MN, Akilimali PZ, Tshibassu PM, et al. Blackwater fever in Congolese children: a report of clinical, laboratory features and risk factors. Malar J. 2013;12:205.
Olupot-Olupot P, Engoru C, Uyoga S, Muhindo R, Macharia A, Kiguli S, et al. High frequency of blackwater fever among children presenting to hospital with severe febrile illnesses in Eastern Uganda. Clin Infect Dis. 2017;64:939–46.
Opoka RO, Waiswa A, Harriet N, John CC, Tumwine JK, Karamagi C. Blackwater fever in Ugandan children with severe anemia is associated with poor post-discharge outcomes: a prospective cohort study. Clin Infect Dis. 2020;70:2247–54.
Conroy AL, Hawkes MT, Leligdowicz A, Mufumba I, Starr MC, Zhong K, et al. Blackwater fever and acute kidney injury in children hospitalized with an acute febrile illness: pathophysiology and prognostic significance. BMC Med. 2022;20:221.
Kamau A, Paton RS, Akech S, Mpimbaza A, Khazenzi C, Ogero M, et al. Malaria hospitalisation in East Africa: age, phenotype and transmission intensity. BMC Med. 2022;20: e28.
Muriuki JM, Mentzer AJ, Mitchell R, Webb EL, Etyang AO, Kyobutungi C, et al. Malaria is a cause of iron deficiency in African children. Nat Med. 2021;27:653–8.
Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, et al. Severe anemia in Malawian children. N Engl J Med. 2008;358:888–99.
Pukrittayakamee S, Looareesuwan S, Keeratithakul D, Davis TM, Teja-Isavadharm P, Nagachinta B, et al. A study of the factors affecting the metabolic clearance of quinine in malaria. Eur J Clin Pharmacol. 1997;52:487–93.
Hanson J, Phu NH, Hasan MU, Charunwatthana P, Plewes K, Maude RJ, et al. The clinical implications of thrombocytopenia in adults with severe falciparum malaria: a retrospective analysis. BMC Med. 2015;13:97.
Leopold SJ, Watson JA, Jeeyapant A, Simpson JA, Phu NH, Hien TT, et al. Investigating causal pathways in severe falciparum malaria: A pooled retrospective analysis of clinical studies. PLoS Med. 2019;16: e1002858.
Church J, Maitland K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med. 2014;12:31.
Phu NH, Day NPJ, Tuan PQ, Mai NTH, Chau TTH, Chuong LV, et al. Concomitant bacteremia in adults with severe falciparum malaria. Clin Infect Dis. 2020;71:e465–70.
PubMed PubMed Central Google Scholar
White NJ, Watson JA, Uyoga S, Williams TN, Maitland KM. Substantial misdiagnosis of severe malaria in African children. Lancet. 2022;400:807.
Aung NM, Nyein PP, Kyi MM, Hanson J. Bacterial coinfection in adults with severe malaria. Clin Infect Dis. 2021;72:535–6.
White NJ.Reply to Aung, et al. Clin Infect Dis. 2021;72:536–8.
Hanson J, Lam SW, Mahanta KC, Pattnaik R, Alam S, Mohanty S, et al. Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis. 2012;206:571–9.
Ishioka H, Ghose A, Charunwatthana P, Maude R, Plewes K, Kingston H, et al. Sequestration and red cell deformability as determinants of hyperlactatemia in falciparum Malaria. J Infect Dis. 2016;213:788–93.
Plewes K, Kingston HWF, Ghose A, Maude RJ, Herdman MT, Leopold SJ, et al. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect Dis. 2017;17:313.
Kingston HWF, Ghose A, Rungpradubvong V, Satitthummanid S, Herdman MT, Plewes K, et al. Cell-free hemoglobin is associated with increased vascular resistance and reduced peripheral perfusion in severe malaria. J Infect Dis. 2020;221:127–37.
Silamut K, Phu NH, Whitty C, Turner GD, Louwrier K, Mai NT, et al. A quantitative analysis of the microvascular sequestration of malaria parasite in the human brain. Am J Pathol. 1999;155:395–410.
Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372:1126–37.
Moghaddam SM, Birbeck GL, Taylor TE, Seydel KB, Kampondeni SD, Potchen MJ. Diffusion-weighted MR imaging in a prospective cohort of children with cerebral malaria offers insights into pathophysiology and prognosis. AJNR Am J Neuroradiol. 2019;40:1575–80.
CAS PubMed PubMed Central Google Scholar
Kampondeni S, Seydel KB, Zhang B, Small DS, Birbeck GL, Hammond CA, et al. Amount of brain edema correlates with neurologic recovery in pediatric cerebral malaria. Pediatr Infect Dis J. 2020;39:277–82.
Anstey NM, Grigg MJ, Rajahram GS, Cooper DJ, William T, Kho S, et al. Knowlesi malaria: Human risk factors, clinical spectrum, and pathophysiology. Adv Parasitol. 2021;113:1–43.
Silamut K, White NJ. Relation of the stage of parasite development in the peripheral blood to prognosis in severe falciparum malaria. Trans R Soc Trop Med Hyg. 1993;87:436–43.
Phu NH, Day NPJ, Diep TS, Ferguson DJP, White NJ. Intraleukocytic malaria pigment and prognosis in severe malaria. Trans R Soc Trop Med Hyg. 1995;89:197–9.
Srinamon K, Watson JA, Silamut K, Intharabut B, Phu NH, Diep PT, et al. The prognostic and diagnostic value of intraleukocytic malaria pigment: an individual patient data pooled meta-analysis of 32,000 patients with severe falciparum malaria in Africa and Asia. Nat Comm, in press.
Poti KE, Sullivan DJ, Dondorp AM, Woodrow CJ. HRP2: transforming malaria diagnosis, but with caveats. Trends Parasitol. 2020;36:112–26.
Mayxay M, Pukritrayakamee S, Chotivanich K, Imwong M, Looareesuwan S, White NJ. Identification of cryptic coinfection with Plasmodium falciparum in patients presenting with vivax malaria. Am J Trop Med Hyg. 2001;65:588–92.
Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212.
Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–7.
Maude RJ, Beare NA, Abu Sayeed A, Chang CC, Charunwatthana P, Faiz MA, et al. The spectrum of retinopathy in adults with Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2009;103:665–71.
Seydel KB, Fox LL, Glover SJ, Reeves MJ, Pensulo P, Muiruri A, et al. Plasma concentrations of parasite histidine-rich protein 2 distinguish between retinopathy-positive and retinopathy-negative cerebral malaria in Malawian children. J Infect Dis. 2012;206:309–18.
Barrera V, MacCormick IJC, Czanner G, Hiscott PS, White VA, Craig AG, et al. Neurovascular sequestration in paediatric P. falciparum malaria is visible clinically in the retina. Elife. 2018;7:e32208.
Dondorp AM, Ince C, Charunwatthana P, Hanson J, van Kuijen A, Faiz MA, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84.
Raja RN. Post-mortem examination in cerebral malaria: a new simple method of demonstrating parasites in the capillaries of the brain. Ind Med Gaz. 1922;57:298–9.
Milner DA Jr, Valim C, Luo R, Playforth KB, Kamiza S, Molyneux ME, et al. Supraorbital postmortem brain sampling for definitive quantitative confirmation of cerebral sequestration of Plasmodium falciparum parasites. J Infect Dis. 2012;205:1601–6.
Gomes MF, Faiz MA, Gyapong JO, Warsame M, Agbenyega T, Babiker A, et al. Pre-referral rectal artesunate to prevent death and disability in severe malaria: a placebo-controlled trial. Lancet. 2009;373:557–66.
WHO. The use of rectal artesunate as a pre- referral treatment for severe P. falciparum malaria. Geneva, World Health Organization, 2022.
Brunner NC, Omoluabi E, Awor P, Okitawutshu J, Tshefu Kitoto A, Signorell A, et al. Prereferral rectal artesunate and referral completion among children with suspected severe malaria in the Democratic Republic of the Congo. Nigeria and Uganda BMJ Glob Health. 2022;7: e008346.
Watson JA, Warsame M, Peto TJ, Onyamboko M, Fanello C, Dondorp AM, et al. Stopping prereferral rectal artesunate - a grave error. BMJ Glob Health. 2022;7: e010006.
Mabey DC, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. 1987;155:1319–21.
Berkley J, Mwarumba S, Bramham K, Lowe B, Marsh K. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg. 1999;93:283–6.
Scott JA, Berkley JA, Mwangi I, Ochola L, Uyoga S, Ndila C, et al. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011;378:1316–23.
Nichols C, Cruz Espinoza LM, von Kalckreuth V, Aaby P, Tayeb M, Ali M, et al. Bloodstream infections and frequency of pretreatment associated with age and hospitalization status in sub-Saharan Africa. Clin Infect Dis. 2015;61(4):S372–9.
Park SE, Pak GD, Aaby P, Adu-Sarkodie Y, Ali M, Aseffa A, et al. The relationship between invasive nontyphoidal Salmonella disease, other bacterial bloodstream infections, and malaria in sub-Saharan Africa. Clin Infect Dis. 2016;62(1):S23-31.
Bruneel F, Tubach F, Mira JP, Houze S, Gibot S, Huisse MG, et al. Imported falciparum malaria in adults: host- and parasite-related factors associated with severity. The French prospective multicenter PALUREA cohort study. Intensive Care Med. 2016;42:1588–96.
Watson JA, Ndila CM, Uyoga S, Macharia A, Nyutu G, Mohammed S, et al. Improving statistical power in severe malaria genetic association studies by augmenting phenotypic precision. Elife. 2021;10: e69698.
Watson JA, Uyoga S, Wanjiku P, Makale J, Nyutu GM, Mturi N, et al. Improving the diagnosis of severe malaria in African children using platelet counts and plasma Pf HRP2 concentrations. Sci Transl Med. 2022;14:5040.
Hendriksen IC, Mwanga-Amumpaire J, von Seidlein L, Mtove G, White LJ, Olaosebikan R, et al. Diagnosing severe falciparum malaria in parasitaemic African children: a prospective evaluation of plasma PfHRP2 measurement. PLoS Med. 2012;9: e1001297.
Hendriksen IC, White LJ, Veenemans J, Mtove G, Woodrow C, Amos B, et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis. 2013;207:351–61.
Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–5.
White NJ, Day NPJ, Ashley EA, Smithuis FM, Nosten FH. Have we really failed to roll back malaria? Lancet. 2022;399:799–800.
John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–9.
Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet. 1990;336:1039–43.
Idro R, Kakooza-Mwesige A, Asea B, Ssebyala K, Bangirana P, Opoka RO, et al. Cerebral malaria is associated with long-term mental health disorders: a cross sectional survey of a long-term cohort. Malar J. 2016;15:184.
Brim R, Mboma S, Semrud-Clikeman M, Kampondeni S, Magen J, Taylor T, et al. Cognitive Outcomes and Psychiatric Symptoms of Retinopathy-Positive Cerebral Malaria: Cohort Description and Baseline Results. Am J Trop Med Hyg. 2017;97:225–31.
Boivin MJ, Mohanty A, Sikorskii A, Vokhiwa M, Magen JG, Gladstone M. Early and middle childhood developmental, cognitive, and psychiatric outcomes of Malawian children affected by retinopathy positive cerebral malaria. Child Neuropsychol. 2019;25:81–102.
Dondorp AM, Hoang MNT, Mer M, Dünser MW, Mohanty S, Nakibuuka J, Management of severe malaria and severe dengue in resource-limited settings., et al. 9. In: Dondorp AM, Dünser MW, Schultz MJ, editors., et al., Sepsis Management in Resource-limited Settings [Internet]. Cham (CH): Springer; 2019. p. 2019.
Chapter Google Scholar
Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–95.
Namutangula B, Ndeezi G, Byarugaba JS, Tumwine JK. Mannitol as adjunct therapy for childhood cerebral malaria in Uganda: a randomized clinical trial. Malar J. 2007;6:138.
Mohanty S, Mishra SK, Patnaik R, Dutt AK, Pradhan S, Das B, et al. Brain swelling and mannitol therapy in adult cerebral malaria: a randomized trial. Clin Infect Dis. 2011;53:349–55.
Ishioka H, Plewes K, Pattnaik R, Kingston HWF, Leopold SJ, Herdman MT, et al. Associations Between Restrictive Fluid Management and Renal Function and Tissue Perfusion in Adults With Severe Falciparum Malaria: A Prospective Observational Study. J Infect Dis. 2020;221:285–92.
Birbeck GL, Herman ST, Capparelli EV, Dzinjalamala FK, Abdel Baki SG, Mallewa M, et al. A clinical trial of enteral Levetiracetam for acute seizures in pediatric cerebral malaria. BMC Pediatr. 2019;19:399.
Gwer SA, Idro RI, Fegan G, Chengo EM, Mpoya A, Kivaya E, et al. Fosphenytoin for seizure prevention in childhood coma in Africa: a randomized clinical trial. J Crit Care. 2013;28:1086–92.
Imwong M, Woodrow CJ, Hendriksen IC, Veenemans J, Verhoef H, Faiz MA, et al. Plasma concentration of parasite DNA as a measure of disease severity in falciparum malaria. J Infect Dis. 2015;211:1128–33.
Sinha I, Ekapirat N, Dondorp AM, Woodrow CJ. Use of a rapid test to assess plasma Plasmodium falciparum HRP2 and guide management of severe febrile illness. Malar J. 2015;14:362.
Rajahram GS, Cooper DJ, William T, Grigg MJ, Anstey NM, Barber BE. Deaths from Plasmodium knowlesi malaria: case series and systematic review. Clin Infect Dis. 2019;69:1703–11.
Barber BE, Grigg MJ, Cooper DJ, van Schalkwyk DA, William T, Rajahram GS, et al. Clinical management of Plasmodium knowlesi malaria. Adv Parasitol. 2021;113:45–76.
Rahimi BA, Thakkinstian A, White NJ, Sirivichayakul C, Dondorp AM, Chokejindachai W. Severe vivax malaria: a systematic review and meta-analysis of clinical studies since 1900. Malar J. 2014;13:481.
Kojom Foko LP, Arya A, Sharma A, Singh V. Epidemiology and clinical outcomes of severe Plasmodium vivax malaria in India. J Infect. 2021;82:231–46.
Phyo AP, Dahal P, Mayxay M, Ashley EA. Clinical impact of vivax malaria: a collection review. PLoS Med. 2022;19: e1003890.
Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax : clinical spectrum, risk factors and pathogenesis. Adv Parasitol. 2012;80:151–201.
Lacerda MV, Fragoso SC, Alecrim MG, Alexandre MA, Magalhães BM, Siqueira AM, et al. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis. 2012;55:e67-74.
Naing C, Whittaker MA, Nyunt Wai V, Mak JW. Is Plasmodium vivax malaria a severe malaria?: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8: e3071.
Douglas NM, Pontororing GJ, Lampah DA, Yeo TW, Kenangalem E, Poespoprodjo JR, et al. Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua. Indonesia BMC Med. 2014;12:217.
Patriani D, Arguni E, Kenangalem E, Dini S, Sugiarto P, Hasanuddin A, et al. Early and late mortality after malaria in young children in Papua. Indonesia BMC Infect Dis. 2019;19:922.
Dini S, Douglas NM, Poespoprodjo JR, Kenangalem E, Sugiarto P, Plumb ID, et al. The risk of morbidity and mortality following recurrent malaria in Papua, Indonesia: a retrospective cohort study. BMC Med. 2020;18:28.
Download references
Acknowledgements
I am a Wellcome Trust Principal Fellow (093956/Z/10/C). I am very grateful to my colleagues in the Mahidol Oxford Research Unit and associated research programmes for all their advice and help.
NJW is a Wellcome Trust Principal Fellow (093956/Z/10/C).
Author information
Authors and affiliations.
Mahidol Oxford Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Nicholas J. White
Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
You can also search for this author in PubMed Google Scholar
Contributions
The author wrote read and approved the final manuscript.
Corresponding author
Correspondence to Nicholas J. White .
Ethics declarations
Ethics approval and consent to participate.
Not relevant.
Consent for publication
Competing interests.
The author declares no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
White, N.J. Severe malaria. Malar J 21 , 284 (2022). https://doi.org/10.1186/s12936-022-04301-8
Download citation
Received : 12 August 2022
Accepted : 23 September 2022
Published : 06 October 2022
DOI : https://doi.org/10.1186/s12936-022-04301-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
- Research and Innovation
- The Abstract
- Audio Abstract Podcast
- Centennial Campus
- Campus Life
- Faculty and Staff
- Awards and Honors
- HR and Finance
- Resilient Pack
- We Are the Wolfpack
- Service and Community
- Red Chair Chats
- News Releases
- In the News
- NC State Experts on 2024 Elections
- NC State Experts Available on Climate
- NC State Experts on Roe v. Wade
- NC State Supply Chain Experts
- Experts on COVID-19
- Hurricane Experts
- About NC State News
- Faculty Support
- Training Program
The Unintended Consequences of Success Against Malaria

For Immediate Release
For decades, insecticide-treated bed nets and indoor insecticide spraying regimens have been important – and widely successful – treatments against mosquitoes that transmit malaria, a dangerous global disease. Yet these treatments also – for a time – suppressed undesirable household insects like bed bugs, cockroaches and flies.
Now, a new North Carolina State University study reviewing the academic literature on indoor pest control shows that as the household insects developed resistance to the insecticides targeting mosquitoes, the return of these bed bugs, cockroaches and flies into homes has led to community distrust and often abandonment of these treatments – and to rising rates of malaria.
In short, the bed nets and insecticide treatments that were so effective in preventing mosquito bites – and therefore malaria – are increasingly viewed as the causes of household pest resurgence.
“These insecticide-treated bed nets were not intended to kill household pests like bed bugs, but they were really good at it,” said Chris Hayes, an NC State Ph.D. student and co-corresponding author of a paper describing the work. “It’s what people really liked, but the insecticides are not working as effectively on household pests anymore.”
“Non-target effects are usually harmful, but in this case they were beneficial,” said Coby Schal, Blanton J. Whitmire Distinguished Professor of Entomology at NC State and co-corresponding author of the paper.
“The value to people wasn’t necessarily in reducing malaria, but was in killing other pests,” Hayes added. “There’s probably a link between use of these nets and widespread insecticide resistance in these house pests, at least in Africa.”
The researchers add that other factors – famine, war, the rural/city divide, and population displacement, for example – also could contribute to rising rates of malaria.
To produce the review, Hayes combed through the academic literature to find research on indoor pests like bed bugs, cockroaches and fleas, as well as papers on malaria, bed nets, pesticides and indoor pest control. The search yielded more than 1,200 papers, which, after an exhaustive review process, was whittled down to a final count of 28 peer-reviewed papers fulfilling the necessary criteria.
One paper – a 2022 survey of 1,000 households in Botswana – found that while 58% were most concerned with mosquitoes in homes, more than 40% were most concerned with cockroaches and flies.
Hayes said a recent paper – published after this NC State review was concluded – showed that people blamed the presence of bed bugs on bed nets.
“There is some evidence that people stop using bed nets when they don’t control pests,” Hayes said.
The researchers say that all hope is not lost, though.
“There are, ideally, two routes,” Schal said. “One would be a two-pronged approach with both mosquito treatment and a separate urban pest management treatment that targets pests. The other would be the discovery of new malaria-control tools that also target these household pests at the same time. For example, the bottom portion of a bed net could be a different chemistry that targets cockroaches and bed bugs.
“If you offer something in bed nets that suppresses pests, you might reduce the vilification of bed nets.”
The study appears in Proceedings of the Royal Society B . The review was supported in part by the Blanton J. Whitmire Endowment at NC State, and grants from the U.S. Department of Housing and Urban Development Healthy Homes program (NCHHU0053-19), the Department of the Army, U.S. Army Contracting Command, Aberdeen Proving Ground, Natick Contracting Division, Ft. Detrick, Maryland (W911QY1910011), and the Triangle Center for Evolutionary Medicine (257367).
-kulikowski-
Note to editors : The abstract of the paper follows.
“Review on the impacts of indoor vector control on domiciliary pests: good intentions challenged by harsh realities”
Authors: Chris Hayes and Coby Schal, NC State University
Published: July 24, 2024 in Proceedings of the Royal Society B
DOI: 10.1098/rspb.2024.0609
Abstract : Arthropod vectored diseases have been a major impediment to societal advancements globally. Strategies to mitigate transmission of these diseases include preventative care (e.g., vaccination), primary treatment, and most notably the suppression of vectors in both indoor and outdoor spaces. The outcomes of indoor vector control (IVC) strategies, such as long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS), are heavily influenced by individual and community-level perceptions and acceptance. These perceptions, and therefore product acceptance, are largely influenced by the successful suppression of non-target nuisance pests such as bed bugs and cockroaches. Adoption and consistent use of LLINs and IRS is responsible for immense reductions in the prevalence and incidence of Malaria. However, recent observations suggest that failed control of indoor pests, leading to product distrust and abandonment, may threaten vector control program success and further derail already slowed progress towards malaria elimination. We review the evidence of the relationship between IVC and nuisance pests and discuss the dearth of research on this relationship. We make the case that the ancillary control of indoor nuisance and public health pests needs to be considered in the development and implementation of new technologies for malaria elimination.
- Designing Healthy and Resilient Societies
- college of agriculture and life sciences
- Department of Entomology and Plant Pathology
- pesticide resistance
More From NC State News

Array Pinpoints Imprinted Genes with Potential Links to Disease

How a ‘Digital Twin’ Can Make Wireless Networks Faster, More Reliable
Nanoscopic Imaging Aids in Understanding Protein, Tissue Preservation in Ancient Bones
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
July 24, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Research sheds light on the role of PTPRK in tissue repair and cancer
by Babraham Institute
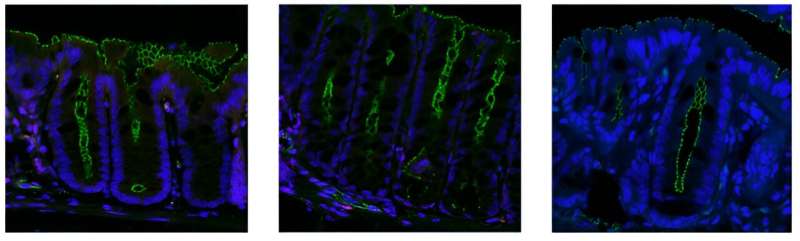
Receptor protein tyrosine phosphatases are cell membrane-localized proteins. They are regulators of cell-cell contacts and are also considered likely to be tumor suppressors, but the specifics of how they function are unknown. A member of this family, PTPRK, is implicated as a tumor suppressor in several cancer types, particularly colorectal cancer, and mutations and genetic events inactivating PTPRK are found in human colorectal cancers. PTPRK has also been linked genetically to celiac disease.
The Sharpe lab at the Babraham Institute investigated the role and signaling mechanisms of PTPRK in cell adhesion , growth factor signaling and tumor suppression in the mouse colon and also in human colorectal cancer cells. Their findings, published in the Journal of Cell Science , are relevant to better understanding the cellular environments that function to repress tumor development as well as understanding the cell interactions that affect repair after injury and potentially cancer metastasis.
Dr. Katie Young, lead author on the paper who undertook this research as a Ph.D. student in the Sharpe lab, said, "Through this work we aimed to investigate the role of PTPRK in the colon, working together several observations in the field and connecting these back to the complex signaling mechanisms behind them. It's vital that we know more about how receptor protein tyrosine phosphatases sense and transmit signals to ensure the healthy growth of our cells, as well as how errors in these mechanisms cause disease."
Using human colorectal cancer cell lines, the team found that the deletion of PTPRK altered the appearance of the cells, compared to control cells where PTPRK was functional, and observed that the knockout cells showed impaired wound-healing response, which was likely to be due to the loss of PTPRK affecting coordinated action by cells and their neighbors and defects in cellular polarization.
Utilizing a mouse line where PTPRK had been deleted, the team uncovered a role for PTPRK in colon repair. When inflammation of the colon (colitis) was stimulated, mice lacking PTPRK showed a more severe response, demonstrating either increased susceptibility to damage or decreased repair following inflammation. The knockout mice also developed larger and more invasive tumors in a colorectal cancer model compared to wild-type controls, confirming that PTPRK has a role in suppressing tumor growth and invasion.
Using a catalytic mutant, where the catalytic function of PTPRK was abolished, and a xenograft model where cancer cells were transplanted into mice, the researchers confirmed the function of PTPRK in suppressing tumor growth and demonstrated that this was independent of the protein's phosphatase activity.
Comparing gene expression profiles between cells with and without PTPRK, the team identified genes that were affected by the loss of PTPRK. These genes are characterized in function as being related to epithelial cell identity (being involved in the epithelial to mesenchymal transition and mesenchymal cell differentiation).
The team hypothesize that PTPRK regulation could be a central factor in giving plasticity in epithelial barriers, such as lines in the intestines, to facilitate epithelial repair while providing a signal to stop the repair response.
Analyzing the xenograft tumor samples, the team quantified tyrosine phosphorylation to determine the signaling mechanisms by which PTPRK suppresses tumor development. Their work suggests that the suppression of epidermal growth factor receptor (EGFR) signaling by PTPRK is a key factor and is mediated separately from its function as a phosphatase.
Dr. Hayley Sharpe, group leader in the Signaling research program at the Institute, said, "The goal of our research was to pull several observations together and begin to fill in the gaps of what we don't know about PTPRK. It has been assumed that PTPs act as tumor suppressors by countering kinase activity by dephosphorylation on oncogenic phosphotyrosine modifications. Therefore, the non-catalytic role of PTPRK in signaling is really intriguing to us and how it achieves this is an important next question to fully understand its role in tumor suppression."
Explore further
Feedback to editors

Conflicting health advice from agencies drives confusion, study finds, but doctors remain most trusted
4 minutes ago
Scientists identify key protein behind spread of shingles virus
7 hours ago

Antisense oligonucleotide treatment shows promise in treating Parkinson's disease progression

Artificial blood vessels could improve heart bypass outcomes
8 hours ago

Exploring the dynamics of combating market-driven epidemics
9 hours ago
Study identifies two critical genes in pancreatic tumors

Exploring consciousness with 'eureka' moments
How epigenetics influence memory formation
10 hours ago
Machine learning shows potential for predicting multiple sclerosis progression
Study finds biosimilars offer improved outcome and lower cost for rheumatoid arthritis treatment
Related stories.
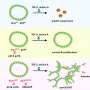
Genetic switches in tumor development may lead to new therapeutic target for colorectal cancer
Nov 29, 2023

Researchers find potential new target against colorectal cancer
Jul 28, 2022

Colon cancer: Curcumin activates tumor suppressive signaling pathway
May 25, 2023

Protein's role in inflammation-related cancer studied
Aug 19, 2019

RAGE as a driver of cell plasticity in aggressive subtype of triple-negative breast cancer
Aug 8, 2023
Study identifies new mechanisms that boost p53 signaling and tumor suppression
Sep 20, 2022
Recommended for you

Electrical currents may make body's cancer-killing cells even better killers
11 hours ago
Immune cell transformation can influence stem cell transplant success in cancer patients
12 hours ago
Self-amplifying mRNA vaccines appear safe in lab and animal tests
How a bacterium supports healing of chronic diabetic wounds

Clinical trial: Fecal matter transplant helps half of patients with GI cancers overcome immunotherapy resistance
13 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 August 2023
Intelligent diagnostic model for malaria parasite detection and classification using imperative inception-based capsule neural networks
- Golla Madhu ORCID: orcid.org/0000-0002-4170-3146 1 ,
- Ali Wagdy Mohamed ORCID: orcid.org/0000-0002-5895-2632 2 , 3 ,
- Sandeep Kautish ORCID: orcid.org/0000-0001-5120-5741 4 ,
- Mohd Asif Shah ORCID: orcid.org/0000-0002-0351-9559 5 , 6 , 7 &
- Irfan Ali ORCID: orcid.org/0000-0002-1790-5450 8
Scientific Reports volume 13 , Article number: 13377 ( 2023 ) Cite this article
4048 Accesses
7 Citations
1 Altmetric
Metrics details
- Epidemiology
Malaria is an acute fever sickness caused by the Plasmodium parasite and spread by infected Anopheles female mosquitoes. It causes catastrophic illness if left untreated for an extended period, and delaying exact treatment might result in the development of further complications. The most prevalent method now available for detecting malaria is the microscope. Under a microscope, blood smears are typically examined for malaria diagnosis. Despite its advantages, this method is time-consuming, subjective, and requires highly skilled personnel. Therefore, an automated malaria diagnosis system is imperative for ensuring accurate and efficient treatment. This research develops an innovative approach utilizing an urgent, inception-based capsule network to distinguish parasitized and uninfected cells from microscopic images. This diagnostic model incorporates neural networks based on Inception and Imperative Capsule networks. The inception block extracts rich characteristics from images of malaria cells using a pre-trained model, such as Inception V3, which facilitates efficient representation learning. Subsequently, the dynamic imperative capsule neural network detects malaria parasites in microscopic images by classifying them into parasitized and healthy cells, enabling the detection of malaria parasites. The experiment results demonstrate a significant improvement in malaria parasite recognition. Compared to traditional manual microscopy, the proposed system is more accurate and faster. Finally, this study demonstrates the need to provide robust and efficient diagnostic solutions by leveraging state-of-the-art technologies to combat malaria.
Similar content being viewed by others

Reducing data dimension boosts neural network-based stage-specific malaria detection
Efficient deep learning-based approach for malaria detection using red blood cell smears
Enhancing parasitic organism detection in microscopy images through deep learning and fine-tuned optimizer
Introduction.
Malaria is a life-threatening disease that involves the Plasmodium parasite, which poses a high death rate. It is transmitted to humans by biting an infected female mosquito with the parasite. Malaria is predominantly a tropical disease since mosquitoes thrive in tropical areas, and it is both preventable and treated. According to the latest Global Malaria Report, there are projected to be around 241 million malaria cases and 627 thousand fatalities worldwide by 2022 1 . Moreover, research by the World Health Organization (WHO) suggests that concerns related to COVID-19 could triple the number of malaria cases 2 , 3 . In response to this global epidemic, the WHO has enacted policies to prevent, treat, eradicate, and monitor malaria 4 . Malaria, a preventable disease, can be controlled and prevented if adequate processes and protocols are used, including early diagnosis of the malarial parasite 4 . Several laboratory techniques, including polymerase chain reaction (PCR), microscopy, and rapid diagnostic test (RDT) are commonly used for investigating malaria using thick or thin blood smears 5 , 6 , 7 , 8 . However, conventional methods tend to rely heavily on manually examining blood smears under a microscope. These methods are time-consuming, subjective, and require highly trained personnel. Additionally, the reliance on clinical experts raises concerns about the consistency and accuracy of the diagnosis. To address these deficiencies, computer-aided diagnostic (CAD) methods for malaria evaluation are being developed to reduce mortality rate 9 . Therefore, automated and accurate diagnostic systems are needed to improve malaria detection. Artificial intelligence has gained more and more attention in the scientific community. It has contributed to improving detection through various diagnostic processes. Most medical imaging analyses now incorporate CAD procedures that leverage deep learning techniques for effective model learning.
However, despite advancements, malaria remains endemic in some areas where the disease is common. Early screening plays a crucial role in detecting malaria and saving lives. Consequently, this motivates us to create faster and more accurate malaria diagnosis procedures. Recently, deep learning architectures have received much attention in terms of research and are the most important method to detect disease automatically and more accurately. These generic deep networks have played a vital role in image classification, detection, and recognition 10 , 11 . In a similar vein, data-driven deep learning (DL) algorithms have surpassed manually constructed feature extraction techniques 12 . A convolutional neural network (CNN) is a type of deep learning model that employs different mechanisms, such as local receptive fields, shared weights, and clustering layers, to leverage information. Its purpose is not limited to extracting features but also extends to generating predictive targets and furnishing actionable predictive models that can effectively aid physicians 10 , 13 . Deep neural networks have shown outstanding performance in computer vision tasks in recent years. This is done using methods like the ResNet-32 network model to identify ductal carcinomas 14 precisely. Despite their effectiveness, CNN suffers from limitations in the modeling of spatial relationships and the lack of an internal representation of the geometrical restrictions on the image data. When these flaws are applied to microscopic cell images, the diagnostic model may be misclassified. The need for a more precise and efficient model arises to improve the performance of detecting and classifying malaria parasites. These challenges have prompted us to develop a rapid and more accurate diagnosis procedure for malaria. The specific hypotheses tested in this study include:
Hypothesis 1
Using the inception neural network will enable the extraction of rich and discriminative features from microscopic images of malaria cells, improving parasite detection and classification accuracy.
Hypothesis 2
The incorporation of the imperative capsule neural network will enhance the modeling of spatial relationships within the images, allowing for a more precise classification of malaria parasites.
By testing these hypotheses, the study aims to demonstrate the superiority of the proposed approach over traditional manual microscopy and other existing methods for malaria diagnosis.
This paper is organized as follows: The relevant research is presented in Section “ Related works ”, and the proposed inception-based imperative capsule neural network is discussed in Section “ Materials and methods ”. Part “ Experimental results ” summarizes and describes the outcomes of this network. Part “ Conclusions ” concludes with the article's conclusions and suggested recommendations for further study.
Related works
Several researchers have demonstrated promising results in medical applications by using data-driven machine learning (ML) and deep learning (DL) models. This study examines contemporary deep-learning applications that elicit key decision-making factors in the diagnosis process. Liang et al. 15 presented a 16-layer CNN to classify the parasitized and uninfected cells in thin blood smears. Features are extracted using a pre-trained AlexNet 16 , and a support vector machine (SVM) is trained on these features, and the model has an average accuracy of 97.37%. However, the transfer learning method achieves only 91.99% accuracy. Bibin et al. 17 proposed and tested a six-layer deep belief network to detect malaria parasites in cell images. Based on their findings, the study achieved 96.4% classification accuracy on a custom dataset using training or test randomization. Dong et al. 18 presented SVM and CNN-based approaches for classifying malaria parasites from cell images. This study attained an accuracy of more than 95% using pre-trained deep learning models such as those used in LeNet 19 , AlexNet 16 , and GoogLeNet 20 . Rajaraman et al. 21 proposed a deep-learning model for malaria parasite detection and classification. The method visualizes the activation maps of each layer and understands the probabilities of the different layers to understand the modeling process. As a result, it obtains an accuracy of 98.61%. Mahdi Postchi et al. 22 surveyed the latest advancements in image analysis and machine-learning techniques for diagnosing malaria through microscopy. Although many machine learning models using traditional features have been developed for image classification and decision-making, these models may lack generalization ability. Sivaramakrishnan et al. 23 suggested a customized CNN model and evaluated the effectiveness of pre-trained and deep-learning CNN models as feature extractors for microscopic images to differentiate between healthy and parasitic blood cells. The model uses surface features to achieve more outstanding results than deep features and applies a level-set-based algorithm to detect and segment red blood cells. This model achieved 98.6% (cell-level) accuracy. Yang et al. 24 presented a fivefold cross-validation for two-step CNN models. In the first step, the model uses an intensity-based iterative Global Mini-mum Screening method to recognize parasites, and then a CNN uses a custom CNN to classify the presence of parasites. The success rate of this method is 93.46%. Vijayalakshmi et al. 25 presented a transfer learning method with a classification accuracy of 93.13% to discriminate between illustrations of malaria-diseased cells and healthy using the VGG16 model and a support vector machine. Madhu et al. 26 proposed an improved dynamic routing process to classify malaria-infected cells from healthy cells using a fully trained capsule network, and the model achieved an accuracy of 98.82%. Loddo et al. 27 used the DenseNet-201 neural network to categorize Plasmodium falciparum life stages into four groups and used two different datasets to assess the robustness of the model. The binary classification accuracy rate was 97.68%, and the multi-classification accuracy rate was 99.40%. Meng et al. 28 proposed a neighborhood correlation graph convolutional network to identify multistage malaria parasites. The model has excellent recognition ability for multistage malaria parasites, outperforming the comparison method by at least 8.67%. Madhu et al. 29 proposed an automated diagnostic model based on deep Siamese capsule arrays for uniquely detecting and classifying malaria parasites. When simplified on the largest test sample (test = 40%), the model achieved an accuracy of 96.61% and 98%, respectively. Ha et al. 30 presented a semi-supervised graph learning framework to solve the problem of identifying apicomplexan parasites. Hybrid graph learning is also used in this approach to explore the relationships between different parasites with and without labels.
In malaria, the Plasmodium parasite causes an acute fever that is carried by female Anopheles mosquitoes. It produces life-threatening sickness if left untreated for a long time, and delaying exact treatment might lead to the development of additional comorbidities. A microscope is currently the most prevalent method for detecting malaria. Consequently, an automated approach to diagnosing malaria is required. This study proposes the development of an urgent, inception-based capsule network for classifying parasitized and uninfected cells from micrographs. These diagnostic models contain neural networks based on the Inception and Imperative Capsule architectures. Using a trained model, such as Inception V3, the first block collects rich characteristics from images of malaria cells. In the second block, a dynamic imperative capsule neural network classifies malaria cells into infected and uninfected red blood cells. The experiment's findings indicate a considerable improvement in recognizing malaria parasites, which contributes to better illness diagnosis and prevention.
By observing the existing challenges, this study aims to develop an automatic diagnostic prototype for classifying malaria parasites from microscopic cell images using the Inception neural network with the Imperative Capsule neural network. The preliminary results of this study are presented as follows:
To develop an innovative approach employing an urgent, inception-based capsule network to recognize parasitized and uninfected cells from microscopic images.
The Inception block extracts rich features from malaria cell images using a pre-trained model, such as Inception V3, which facilitates efficient representation learning to recognize the parasites.
The dynamic imperative capsule neural network is utilized to classify microscopic images into parasitized and healthy cells, enabling the detection of malaria parasites.
To compute routing by agreement among low-level and higher-level capsules that can be used to predict malaria cells and classify them into parasitized and uninfected cells using L2-Norm.
This study underscores the importance of leveraging state-of-the-art technologies to combat malaria by providing a robust and efficient diagnostic solution.
Materials and methods
Dataset collection.
Images of thin blood smears containing two distinct strains of malaria—one infected and the other not—were used in the study. These samples were gathered from patients and healthy controls who had Plasmodium falciparum infections, and they were stored at the National Institutes of Health (NIH) repository, which is open to the public for study 23 . The collection includes 13,779 images of parasites and 13,779 images of uninfected cells, totaling 27,558 images of labeled and segmented cells from thin Giemsa-stained blood smear slides. Figure 1 offers some parasitic and uninfected cell images to visualize their physical traits.
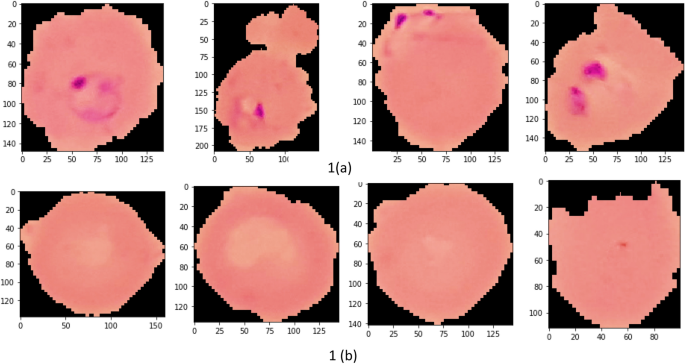
Illustration of sample malaria cell images: ( a ) Infected images; ( b ) Uninfected images (without parasites).
k-fold cross-validation (CV) test
The dataset contains 27,558 blood cell images with malaria-positive and negative samples, which were evaluated in our study for data sample training and testing, and used k-folds (k = 10, 20, 30, 40, 50) Cross-validation to evaluate the proposed model. As shown in Table 1 , the dataset is split into training and testing subsets.
Inception neural network and the imperative capsule neural network
Geoffrey Hinton et al. 31 motivated this research by addressing the limitations of traditional CNNs by proposing inception-based capsule neural networks, which require small data but have higher computational complexity.
This research develops an inception-based imperative capsule neural network for malaria detection, and its basic architecture is shown in Fig. 2 , which is similar to the architecture advocated for image classification problems by Sabour et al. 31 . According to Fig. 2 , input is first routed through fully connected inception blocks, which receive the parasitized and uninfected portions of the cell images as input and extract features on the parasitized and uninfected portions of the cell images. The inception block's output is used as the primary capsule layer's input. The primary and higher capsule layers utilize an imperative routing mechanism to learn the captured features by discerning the spatial orientation of the parasites on the extracted features. After multiple iterations, the resulting output is a feature vector with a length equivalent to the probability of the interval [0, 1], which preserves the object's pose information, minimizing the information loss caused by the feature vector extraction. This feature vector is then used to classify a test sample as infected or healthy cells, aiding in its classification.
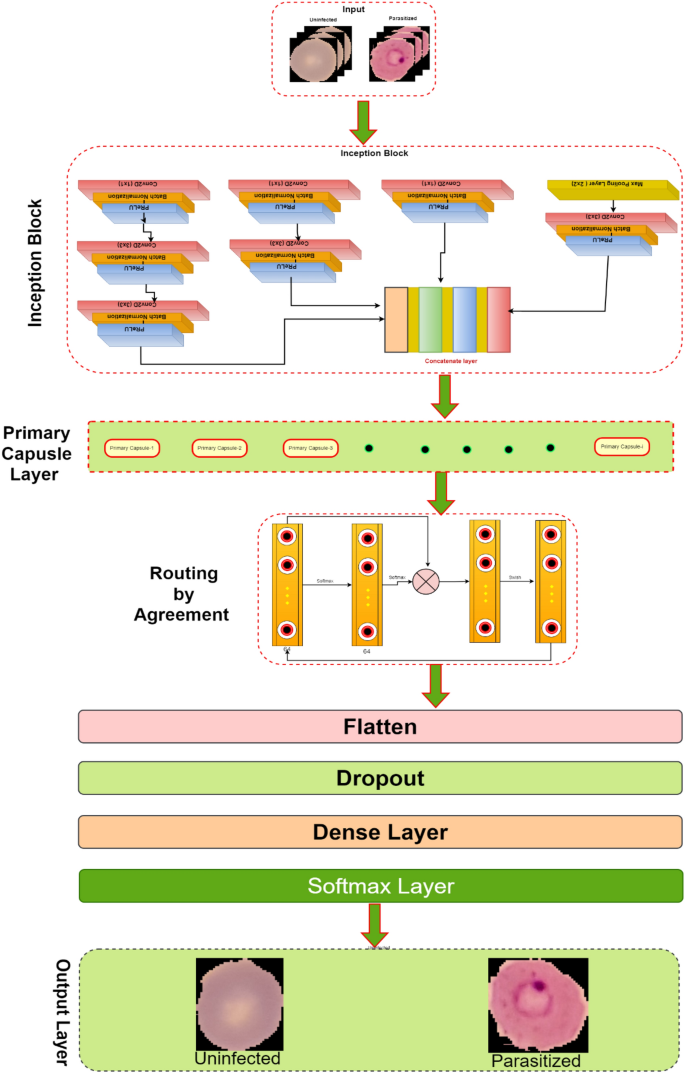
The proposed architecture of Inception-based capsule neural network.
Inception neural network block
In 2015, Google introduced a module for GoogleNet 32 , also known as Inception V3, a convolutional neural network that helps us with image analysis and object detection.
Convolutional layers are frequently employed in convolutional neural networks (CNNs) to extract information from images of malaria blood cells. The CNN's initialization block, which is made up of parallel convolutional layers with filters and kernels of various sizes, extracts feature from various scales to obtain multi-view information on parasites and healthy cells. The structure of the inception block, which is used to extract characteristics at various scales, is shown in Fig. 3 . To extract features at various sizes, this block has four parallel convolutional layers with various kernels (1 × 1, 3 × 3, and 3 × 3). A max-pooling layer with a kernel size of 2 × 2, a convolution layer with a kernel size of 1 × 1, and a batch normalizing layer make up the final parallel convolutional layer. Each parallel layer's computational cost and channel count can be decreased by using a 1 × 1 convolutional layer, and the model's computational cost can be decreased by employing a 3 × 3 max-pooling layer. The output feature maps of each of the four simultaneous convolutional layers are combined after computation to produce new feature maps that are used as the input for the capsule network.
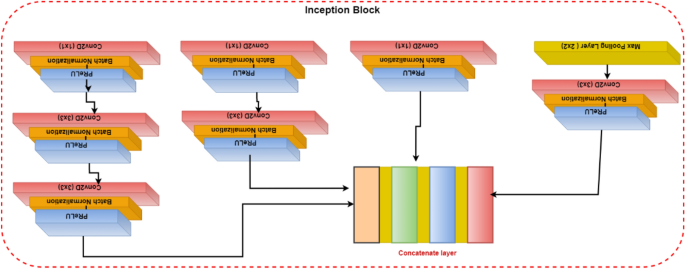
Illustration of the inception block.
Capsule networks block
To classify the items in the MNIST dataset, Sabour et al. 31 presented a capsule network (CapsNet). It uses a neural network to produce an output vector that includes both a scalar and a vector encoding the features of the objects in the image. In our experiment, these capsule networks are trained by carefully adjusting the number of rounds in the dynamic routing algorithm. Using Parametric ReLU (PReLU), it is possible to investigate the behavior of nonlinear activations during dynamic routing 33 . The presence of features in the form of vectors containing low-level entity instantiation parameters is estimated using the principal capsule layer. CapsNet transforms the scalar output using feature detectors in this layer, then passes the vector output of the capsules to the following layer using a modified routing method 31 . Because parameter tuning is critical for better network learning and faster convergence, proper initialization is used to start the routing procedure with kernel initializer before the primary capsule layer; the dynamic routing algorithm is activated with Glorot-normalization 34 . Each capsule, \(i\) has an activity vector \({u}_{i}\in R\) in the layer of \(l,\) which captures information about the features extracted from an entity (i.e., blood cell image). The output of the activity vector \({u}_{i}\) of the \(i\) th level capsule is fed as data into the next level layer, i.e., \(l+1\) layer. The \({j}{\text{th}}\) layer capsules of layer \(l+1\) will get data from \({u}_{i}\) and compute the product weight matrix \({W}_{ij}^{T}\) . The results are stored in the form of \({\widehat{u}}_{(j|i)}.\) This vector is the layer of capsules \(i\) at level \(l\) layer, which is the transformation of the entity represented by capsule \(j\) at the level of \(l+1\) . Then apply the transformation matrix \({W}_{ij}^{T}\) to capsule output \({u}_{i}\) of the previous layer, as shown in Eq. ( 1 ).
In Eq. ( 1 ), capsule \(i\) is the primary capsule layer, \(j\) is the higher-level capsule layer, and \({u}_{i}\) is the output of the capsule network of the upper layer and \({W}_{ij}^{T}\) is the learnable weighted matrix between the \({i}{\text{th}}\) capsule to \({j}{\text{th}}\) capsule. Which is multiplied by each output vector and the coupling coefficient \({C}_{ij}\) is added to the linear sum stage. Then the capsules are in the higher level, which is filled with the sum of the output vector in the lower-level layer, and we add it with a coupling coefficient \({C}_{ij}\) which is computed during the routing method shown in Eq. ( 2 ).
In dynamic routing, the coupling coefficient is determined by Eq. ( 2 ). In the process of calculating \({S}_{j}\) in forward propagation, \({W}_{ij}^{T}\) is set to a random value, \({a}_{ij}\) is initialized to zero, \({u}_{i}\) is the output of the previous layer, and then compute a weighted sum \({S}_{j}\) with weights \({C}_{ij}\) (the sum of these coefficients is equal to one) and it is denoted as follows:
The squashing function map of \({S}_{j}\) yields the output vector \({v}_{j},\) which is obtained is defined as follows:
The squashing function, defined by Eq. ( 4 ), ensures that short vectors are reduced to fewer dimensions near zero while long vectors are scaled to unit length, thus introducing nonlinearity to the capsule network. The total input Sj processed by the jth dimensional capsule array contributes to the coupling coefficient Cij. An activation function PReLU is applied to update the coupling coefficients, instead of the squashing function, by operating on Sj. During the iterative learning phase, these coupling coefficients are updated using Eq. ( 5 ), which proceeds as follows:
In Eq. ( 5 ), \({a}_{ij}\) is a parameter used as a weighted proxy, which means that it gives higher weights to appropriate predictions, and it starts at zero and is modified as the training progress.
However, it is initialized with the current input weights to improve the learning method by reducing the computational cost and improving the predictive ability. The number of routing iterations (n = 3) is used as a hyperparameter allowing one to choose a specific number of iterations during the training (here, epochs = 100) period, and the details of this network parameters are shown in Table 2 . The learning period is evaluated by evaluating the convergence, and our model is repeated for only three iterations. Figure 4 depicts the comprehensive learning curves for iterations over 100 epochs.
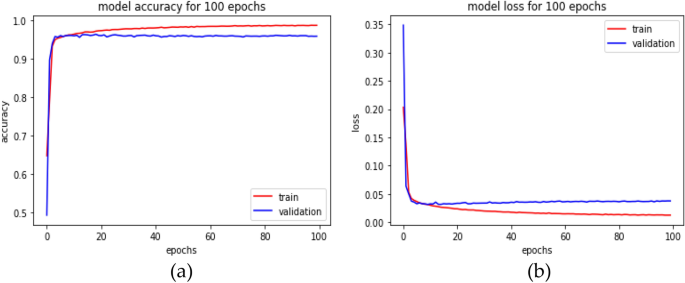
An inception-based capsule network with a router in 3 iterations, depicted as ( a ) accuracy curves and ( b ) loss decay curves.
PReLU activations are utilized during the routing by agreement process to improve the understanding of feature invariance in the captured images of malaria cells. In a conventional capsule network, the squash activation function is typically used as a non-linearity. However, using PReLU as a non-linearity is believed to lead to better generalization and convergence over time. The last layer of the network comprises two capsules (parasitized and uninfected cells) reflecting the probability of the interval [0, 1] and the position information of the object, preserving the pose information to reduce information loss caused by the extracted feature vector. This enables the classification of test samples into either parasitized or uninfected cells, thus aiding in cell feeding.
Loss function
Our current loss function 31 also includes the mean squared error rate (MSE) alongside the marginal loss. Change the settings for faster convergence and add proper model regularization and noise addition when training the classification model with a value set to 0.45.
In Eq. ( 6 ), \({m}^{+}\) and \({m}^{-}\) are the category prediction values, \(\sigma \) is the balance coefficient, \({T}_{x} \mathrm{is \, the \, label \, of \, category}, \) and classification probability vector \(\Vert {v}_{x}\Vert \) is the size. For this study, the default values are set as \({m}^{+}=0.85 \& {m}^{-}=0.15\) , \(\sigma =0.45\) . The total loss function, in this case, refers to the loss of capsules representing both malaria-parasitized and uninfected classes.
Experimental results
This section describes the proposed model's implementation in-depth and thoroughly analyses how well it performs under various restrictions. The proposed network was evaluated against front-line classification models created by several authors, which were pre-trained using NIH malaria datasets 23 and other private datasets to assess whether red blood cells are parasitized or not. According to Table 3 , the proposed model for malaria parasite identification and classification performed well on the NIH malaria dataset, along with the comparison findings. It is important to note that most models typically exhibit low performance on this dataset. Although their weights can handle common classification datasets, they frequently fall short because of ineffective feature extraction brought on by too much depth. Instead, the Inception-based capsule network model classifies parasitized and uninfected cells accurately during the diagnostic process by utilizing external knowledge to produce rich characteristics. On international benchmarks, the suggested model performs noticeably better.
As stated in the Table 4 , our model is assessed for layer-wise testing cell images, varying from training to 80% and testing to 20%.
In this analysis, experiments are conducted on various distributions, and the suggested network's implementation, as shown in Table 4 , achieves an accuracy of 99.35% and an AUC score of at least 99.73% at a test ratio of 20%. Table 4 shows the models' overall generality as measured by various standard classification metrics, including accuracy score, AUC–ROC, sensitivity, and specificity. Limiting diagnostic power does not assess the likelihood that a certain patient will acquire a disease, but it does affect diagnostic accuracy, even though they choose sensitivity and specificity. Table 5 displays the effectiveness of the suggested capsule array at various nonlinearity levels. Compared to the performance of cutting-edge pre-trained models, the generalization distribution for the training and test samples is 80% to 20%.
The performance metrics for every deep learning architecture are compiled in Table 5 . The proposed malaria detection algorithm outperforms the compared deep learning models in terms of performance. The results showed an accuracy of more than 99.35%, an AUC score of 99.73%, and an F1 score of 99.36%. The accuracy score is a well-known metric with a domain that is invariant to general utility; hence it is imperative to note. As a result, the effectiveness of the suggested model is assessed using various measuring techniques. The model was created to be assessed by segregating partition samples that vary from 10 to 50%, ensuring that the model is adequately generalized. Figure 5 displays the predicted results of the suggested model on images of malarial cells. The true value is shown on the x-axis, and the model forecast is shown on the y-axis.
Illustration of some prediction results of the proposed model.
Time complexity analysis
According to our study, the learning model was trained for 100 epochs to assess the time complexity of the model. The results show that our model takes around 33.8667 min for training and 3 s for complete testing, which is less than all the compared models. This study addresses the urgent need for automated malaria detection and classification. It proposes a novel approach based on integrating inception and imperative capsule neural networks. This research has the potential to significantly improve malaria diagnosis, contributing to more effective disease management and prevention. Additionally, the study contributes to the growing field of deep learning in medical image analysis. It showcases the applicability of advanced neural network architectures to address critical healthcare challenges.
Conclusions
This research develops a deep-learning approach by combining the imperative capsule neural network with the inception neural network to distinguish between malaria-parasitized and uninfected cells. This enhances the classification accuracy of identifying malaria parasites from photographs of blood cells. With well-chosen parameters, the capsule model can efficiently finish the procedure for classifying uninfected cells or parasites into different categories. Models with different loss parameters are compared to the proposed model, and the results show that the model's performance can be increased by adjusting the loss parameters. The proposed network achieves higher classification accuracy while analyzing blood cell images for malaria than competing deep learning methods. Under the worst-case scenario (50/50 split), the model obtains an accuracy of 98.10% on the test, while on the 20% split, it achieves an accuracy of 99.355%. These experimental results are helpful since the developed model is robust and flexible and has outperformed competing models. In the work's future scope, the model may be utilized to recognize parasite species and stages in thin blood smears. This research opens opportunities for future advancements in malaria diagnosis and surveillance, including using mobile and portable imaging devices for point-of-care testing.
Data availability
The data that support the findings of this study are openly available in the National Library of Medicine (NLM)—Malaria Data: https://lhncbc.nlm.nih.gov/LHC-research/LHC-projects/image-processing/malaria-datasheet.html and reference number Ref. 23 .
https://www.who.int/news-room/fact-sheets/detail/malaria .
Alnussairi, M. H. D. & İbrahim, A. A. Malaria parasite detection using deep learning algorithms based on (CNNs) technique. Comput. Electr. Eng. 103 , 108316 (2022).
Article Google Scholar
Chakradeo, K., Delves, M. & Titarenko, S. Malaria parasite detection using deep learning methods. Int. J. Comput. Inf. Eng. 15 (2), 175–182 (2021).
Google Scholar
Fact Sheet about MALARIA. https://www.who.int/news-room/fact-sheets/detail/malaria . Accessed 26 Nov 2022.
Devi, S. S., Roy, A., Singha, J., Sheikh, S. A. & Laskar, R. H. Malaria infected erythrocyte classification based on a hybrid classifier using microscopic images of thin blood smear. Multimed. Tools Appl. 77 (1), 631–660 (2018).
Mfuh, K. O. et al. A comparison of thick-film microscopy, rapid diagnostic test, and polymerase chain reaction for accurate diagnosis of Plasmodium falciparum malaria. Malar. J. 18 (1), 1–8 (2019).
Poostchi, M., Silamut, K., Maude, R. J., Jaeger, S. & Thoma, G. Image analysis and machine learning for detecting malaria. Transl. Res. 194 , 36–55 (2018).
Article PubMed PubMed Central Google Scholar
Hanscheid, T. & Valadas, E. Malaria diagnosis. Am. J. Trop. Med. Hyg. 61 , 179. https://doi.org/10.4269/ajtmh.1999.61.179 (1999).
Article CAS PubMed Google Scholar
Alonso-Ramírez, A. A. et al. Classifying parasitized and uninfected malaria red blood cells using convolutional-recurrent neural networks. IEEE Access 10 , 97348–97359 (2022).
Krizhevsky, A., Ilya Sutskever, S. & Geoffrey, E. H. ImageNet classification with deep convolutional neural networks. Commun. ACM 60 (6), 84–90 (2017).
Redmon, J., Divvala, S., Girshick, R. & Farhadi, A. You only look once: Unified, real-time object detection. in Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition , 779–788 (2016).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521 (7553), 436–444 (2015).
Article ADS CAS PubMed Google Scholar
Razzak, M. I., Naz, S. & Zaib, A. Deep Learning for Medical Image Processing: Overview, Challenges, and the Future 323–350 (Springer, 2018).
Praveen, S. P., Srinivasu, P. N., Shafi, J., Wozniak, M. & Ijaz, M. F. ResNet-32 and FastAI for diagnoses of ductal carcinoma from 2D tissue slides. Sci. Rep. 12 (1), 20804 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Liang, Z. et al . CNN-based image analysis for malaria diagnosis. in IEEE International Conference on Bioinformatics and Biomedicine, IEEE , 493–496 (2016).
Krizhevsky, A., Sutskever, I. & Hinton, G. E. ImageNet classification with deep convolutional neural networks. in Proceedings of the 25th International Conference on Neural Information Processing Systems, Volume 1 (NIPS'12) , 1097–1105 (2012).
Bibin, D., Nair, M. S. & Punitha, P. Malaria parasite detection from peripheral blood smear images using deep belief networks. IEEE Access 5 , 9099–9108 (2017).
Dong, Y. et al . Evaluations of deep convolutional neural networks for automatic identification of malaria-infected cells. in EMBS International Conference on Biomedical & Health Informatics, IEEE , 101–104 (2017).
Lecun, Y., Bottou, L., Bengio, Y. & Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 86 (11), 2278–2324 (1998).
Szegedy, C. et al . Going deeper with convolutions. in Proceedings of the 2015 (CVPR) , 1–9 (2015).
Sivaramakrishnan, R., Antani, S. & Jaeger, S. Visualizing deep learning activations for improved malaria cell classification. Med. Inf. Healthc. 1 , 40–47 (2017).
Poostchi, M., Silamut, K., Maude, R. J., Jaeger, S. & Thoma, G. Image analysis and machine learning for detecting malaria. Transl. Res. 194 (6), 36–55 (2018).
Sivaramakrishnan, R. et al. Pre-trained convolutional neural networks as feature extractors toward improved malaria parasite detection in thin blood smear images. PeerJ 6 , e4568 (2018).
Yang, F. et al. Deep learning for smartphone-based malaria parasite detection in thick blood smears. IEEE J. Biomed. Health Inform. 24 (5), 1427–1438 (2019).
Article PubMed Google Scholar
Vijayalakshmi, A. & Rajesh Kanna, B. Deep learning approach to detect malaria from microscopic images. Multimed. Tools Appl. 79 (21), 1–21 (2020).
Madhu, G. et al. Imperative dynamic routing between capsules network for malaria classification. Comput. Mater. Contin. 68 (1), 903–919 (2021).
Loddo, A., Fadda, C. & Di Ruberto, C. An empirical evaluation of convolutional networks for malaria diagnosis. J. Imaging 8 , 3. https://doi.org/10.3390/jimaging8030066 (2022).
Meng, X., Ha, Y. & Tian, J. Neighbor correlated graph convolutional network for multi-stage malaria parasite recognition. Multimed. Tools Appl. 81 , 11393–11414. https://doi.org/10.1007/s11042-022-12098-6 (2022).
Madhu, G. et al. DSCN-net: A deep Siamese capsule neural network model for automatic diagnosis of malaria parasites detection. Multimed. Tools Appl. 81 , 34105–34127. https://doi.org/10.1007/s11042-022-13008-6 (2022).
Ha, Y., Meng, X., Du, Z., Tian, J. & Yuan, Y. Semi-supervised graph learning framework for apicomplexan parasite classification. Biomed. Signal Process. Control 81 , 104502. https://doi.org/10.1016/j.bspc.2022.104502 (2022).
Sabour, S., Frosst, N. & Hinton, G. E. Dynamic routing between capsules. Adv. Neural Inf. Process. Syst. 1 , 3856–3866 (2017).
Szegedy, C. et al . Going deeper with convolutions. in Proc. CVPR 2015 , 1–9 (2015).
He, K., Zhang, X., Ren, S. & Sun, J. Delving deep into rectifiers: Surpassing human-level performance on imagenet classification. in Proceedings of the IEEE International Conference on Computer Vision , 1026–1034 (2015).
Glorot, X. & Bengio, Y. Understanding the difficulty of training deep feedforward neural networks. in Proceedings of the Thirteenth International Conference on Artificial Intelligence and Statistics , 249–256 (2010).
Das, D. K., Maiti, A. K. & Chakraborty, C. Automated system for characterization and classification of malaria-infected stages using light microscopic images of thin blood smears. J. Microsc. 257 (3), 238–252 (2015).
Díaz, G., González, F. A. & Romero, E. A semi-automatic method for quantification and classification of erythrocytes infected with malaria parasites in microscopic images. J. Biomed. Inform. 42 (2), 296–307 (2009).
Gopakumar, G. P. et al. Convolutional neural network-based malaria diagnosis from focus stack of blood smear images acquired using custom-built slide scanner. J. Biophoton. 11 (3), e201700003 (2018).
Rahman, A. et al . Improving malaria parasite detection from red blood cell using deep convolutional neural networks. (2019). arXiv:1907.10418 .
Download references
Author information
Authors and affiliations.
Department of Information Technology, VNR Vignana Jyothi Institute of Engineering and Technology, Hyderabad, Telangana, 500090, India
Golla Madhu
Operations Research Department, Faculty of Graduate Studies for Statistical Research, Cairo University, Giza, 12613, Egypt
Ali Wagdy Mohamed
Applied Science Research Center, Applied Science Private University, Amman, Jordan
LBEF Campus (Asia Pacific University of Technology & Innovation, Malaysia), Kathmandu, 44600, Nepal
Sandeep Kautish
College of Business and Economics, Kabridahar University, Po Box 250, Kabridahar, Ethiopia
Mohd Asif Shah
School of Business, Woxsen University, Kamkole, Sadasivpet, Hyderabad, 502345, Telangana, India
Division of Research and Development, Lovely Professional University, Phagwara, 144001, Punjab, India
Department of Statistics & Operations Research, Aligarh Muslim University, Aligarh, 202002, India
You can also search for this author in PubMed Google Scholar
Contributions
G.M. conceived and designed the experiments, performed the experiments, and prepared figures and/or tables. A.W.M., S.K., M.A.S. and I.A. supervised the study, analyzed the results, and provided insightful suggestions for the manuscript. All authors have read and authored or reviewed drafts of the paper and approved the final draft.
Corresponding author
Correspondence to Mohd Asif Shah .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Madhu, G., Mohamed, A.W., Kautish, S. et al. Intelligent diagnostic model for malaria parasite detection and classification using imperative inception-based capsule neural networks. Sci Rep 13 , 13377 (2023). https://doi.org/10.1038/s41598-023-40317-z
Download citation
Received : 13 April 2023
Accepted : 08 August 2023
Published : 17 August 2023
DOI : https://doi.org/10.1038/s41598-023-40317-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee for the Study on Malaria Prevention and Control; Oaks SC Jr., Mitchell VS, Pearson GW, et al., editors. Malaria: Obstacles and Opportunities. Washington (DC): National Academies Press (US); 1991.

Malaria: Obstacles and Opportunities.
- Hardcopy Version at National Academies Press
1 Conclusions and Recommendations
- DEFINING THE PROBLEM
The outlook for malaria control is grim. The disease, caused by mosquito-borne parasites, is present in 102 countries and is responsible for over 100 million clinical cases and 1 to 2 million deaths each year. Over the past two decades, efforts to control malaria have met with less and less success. In many regions where malaria transmission had been almost eliminated, the disease has made a comeback, sometimes surpassing earlier recorded levels. The dream of completely eliminating malaria from many parts of the world, pursued with vigor during the 1950s and 1960s, has gradually faded. Few believe today that a global eradication of malaria will be possible in the foreseeable future.
Worldwide, the number of cases of malaria caused by Plasmodium falciparum , the most dangerous species of the parasite, is on the rise. Drug-resistant strains of P. falciparum are spreading rapidly, and there have been recent reports of drug resistance in people infected with P. vivax , a less virulent form of the parasite. Furthermore, mosquitoes are becoming increasingly resistant to insecticides, and in many cases, have adapted so as to avoid insecticide-treated surfaces altogether.
In large part because of the spread of drug and insecticide resistance, there are fewer tools available today to control malaria than there were 20 years ago. In many countries, the few remaining methods are often applied inappropriately. The situation in many African nations is particularly dismal, exacerbated by a crumbling health infrastructure that has made the implementation of any disease control program difficult.
Malaria cases among tourists, business travelers, military personnel, and migrant workers in malarious areas have been increasing steadily in the last several years, posing new concerns that the disease will be introduced to currently nonmalarious areas. Recent epidemics have claimed tens of thousands of lives in Africa, and there is an increasing realization that malaria is a major impediment to socioeconomic development in many countries. Unless practical, cost-effective strategies can be developed and successfully implemented, malaria will continue to exact a heavy toll on human life and health around the world.
Although often considered a single disease, malaria is more accurately viewed as many diseases, each shaped by subtle interactions of biologic, ecologic, social, and economic factors. The species of parasite, the behavior of the mosquito host, the individual's immune status, the climate, human activities, and access to health services all play important roles in determining the intensity of disease transmission, who will become infected, who will get sick, and who will die.
Gem miners along the Thailand-Cambodia border, American tourists on a wildlife safari in East Africa, villagers living on the central highlands in Madagascar, residents of San Diego County, California, a young pregnant woman in Malawi, Swiss citizens living near Geneva International Airport, children in Africa south of the Sahara, and a U.S. State Department secretary in Tanzania seem to have little in common, yet they are all at risk of contracting malaria. Because of the disease's variable presentations, each will be affected differently, as illustrated below.
- For the hundreds of thousands of Thai seasonal agricultural workers who travel deep into the forest along the Thailand-Cambodia border to mine for gems, malaria is the cost of doing business. These young men are exposed to aggressive forest mosquitoes, and within two to three weeks after arriving, almost every miner will get malaria. Many gem miners seek medications to prevent and self-treat mild cases of the disease. But because malaria in this part of the world is resistant to most antimalarial drugs, the few effective drugs are reserved for the treatment of confirmed cases of malaria. To complicate matters, there are no health services in the forest to treat patients, and the health clinics in Thailand are overburdened by the high demand for treating those with severe malaria, most of whom are returning gem miners. A similar scenario involving over 400,000 people exists among gold miners in Rondonia, Brazil.
- Each year, over seven million U.S. citizens visit parts of the world where malaria is present. Many, at the recommendation of their travel agent or physician, take antimalarial medications as a preventive measure, but a significant number do not. Tourists and other travelers who have never been exposed to malaria, and therefore have never developed protective immunity, are at great risk for contracting severe disease. Ironically, it is not the infection itself that poses the biggest danger, but the chance that treatment will be delayed because of misdiagnosis upon the individual's return to the United States. Most U.S. doctors have never seen a patient with malaria, are often confused by the wide array of symptoms, and are largely unaware that malaria in a nonimmune person can be a medical emergency, sometimes rapidly fatal.
- Prior to 1950, malaria was the major cause of death in the central highlands of the African island nation of Madagascar. In the late 1950s, an aggressive program of indoor insecticide spraying rid the area of malaria-carrying mosquitoes, and malaria virtually disappeared. By the 1970s, confident of a victory in the battle against malaria, Madagascar began to phase out its spraying program; in some areas spraying was halted altogether. In the early 1980s, the vector mosquitoes reinvaded the central highlands, and in 1986 a series of devastating epidemics began. The older members of the population had long since lost the partial immunity they once had, and the younger island residents had no immunity at all. During the worst of the epidemics, tens of thousands of people died in one three-month period. The tragedy of this story is that it could have been prevented. A cheap antimalarial drug, chloroquine, could have been a powerful weapon in Madagascar, where drug resistance was not a significant concern. Because of problems in international and domestic drug supply and delivery, however, many people did not receive treatment and many died. In the last 18 months, surveillance has improved, spraying against the mosquito has resumed, and more effective drug distribution networks have been established. Malaria-related mortality has declined sharply as a result.
- Malaria, once endemic in the southern United States, occurs relatively infrequently. Indeed, there have been only 23 outbreaks of malaria since 1950, and the majority of these occurred in California. But for each of the past three years, the San Diego County Department of Health Services has had to conduct an epidemiologic investigation into local transmission of malaria. An outbreak in the late summer of 1988 involved 30 persons, the largest such outbreak in the United States since 1952. In the summer of 1989, three residents of San Diego County—a migrant worker and two permanent residents—were diagnosed with malaria; in 1990, a teenager living in a suburb of San Diego County fell ill with malaria. All of the cases were treated successfully, but these incidents raise questions about the possibility of new and larger outbreaks in the future. Malaria transmission in San Diego County (and in much of California) is attributed to the presence of individuals from malaria-endemic regions who lack access to medical care, the poor shelter and sanitation facilities of migrant workers, and the ubiquitous presence of Anopheles mosquitoes in California.
- A 24-year-old pregnant Yao woman from the Mangochi District in Malawi visited the village health clinic monthly to receive prenatal care. While waiting to be seen by the health provider, she and other women present listened to health education talks which were often about the dangers of malaria during pregnancy, and the need to install screens around the house to keep the mosquitoes away, to sleep under a bednet, and to take a chloroquine tablet once a week. Toward the end of her second trimester of pregnancy, the woman returned home from her prenatal visit with her eight tablets of chloroquine wrapped in a small packet of brown paper. She promptly gave the medicine to her husband to save for the next time he or one of their children fell ill. The next week she developed a very high malarial fever and went into labor prematurely. The six-month-old fetus was born dead.
- Over a two-week period in the summer of 1989, five Swiss citizens living within a mile of Geneva International Airport presented at several hospitals with acute fever and chills. All had malaria. Four of the five had no history of travel to a malarious region; none had a history of intravenous drug use or blood transfusion. Apart from their symptoms, the only thing linking the five was their proximity to the airport. A subsequent epidemiologic investigation suggested that the malaria miniepidemic was caused by the bite of stowaway mosquitoes en route from a malaria- endemic country. The warm weather, lack of systematic spraying of aircraft, and the close proximity of residential areas to the airport facilitated the transmission of the disease.
- Malaria is a part of everyday life in Africa south of the Sahara. Its impact on children is particularly severe. Mothers who bring unconscious children to the hospital often report that the children were playing that morning, convulsed suddenly, and have been unconscious ever since. These children are suffering from the most frequently fatal complication of the disease, cerebral malaria. Other children succumb more slowly to malaria, becoming progressively more anemic with each subsequent infection. By the time they reach the hospital, they are too weak to sit and are literally gasping for breath. Many children are brought to hospitals as a last resort, after treatment given for “fever” at the local health center has proved ineffective. Overall, children with malaria account for a third of all hospital admissions. A third of all children hospitalized for malaria die. In most parts of Africa, there are no effective or affordable options to prevent the disease, so children are at high risk until they have been infected enough times to develop a partial immunity.
- A 52-year-old American woman, the secretary to the U.S. ambassador in Tanzania, had been taking a weekly dose of chloroquine to prevent malaria since her arrival in the country the year before. She arrived at work one morning complaining of exhaustion, a throbbing headache, and fever. A blood sample was taken and microscopically examined for malaria parasites. She was found to be infected with P. falciparum , and was treated immediately with high doses of chloroquine. That night, she developed severe diarrhea, and by morning she was found to be disoriented and irrational. She was diagnosed as having cerebral malaria, and intravenous quinine treatment was started. Her condition gradually deteriorated—she became semicomatose and anemic, and approximately 20 percent of her red blood cells were found to be infected with malaria parasites. After continued treatment for several days, no parasites were detected in her blood. Despite receiving optimal care, other malaria-related complications developed and she died just nine days after the illness began. The cause of death: chloroquine-resistant P. falciparum .
These brief scenarios give a sense of the diverse ways that malaria can affect people. So fundamental is this diversity with respect to impact, manifestation, and epidemiology that malaria experts themselves are not unanimous on how best to approach the disease. Malariologists recognize that malaria is essentially a local phenomenon that varies greatly from region to region and even from village to village in the same district. Consequently, a single global technology for malaria control is of little use for specific conditions, yet the task of tailoring strategies to each situation is daunting. More important, many malarious countries do not have the resources, either human or financial, to carry out even the most meager efforts to control malaria.
These scenarios also illustrate the dual nature of malaria as it affects U.S. policy. In one sense, it is a foreign aid issue; a devastating disease is currently raging out of control in vast, heavily populated areas of the world. In another sense, malaria is of domestic public health concern. The decay of global malaria control and the invasion of the parasite into previously disease-free areas, coupled with the increasing frequency of visits to such areas by American citizens, intensify the dangers of malaria for the U.S. population. Tourists, business travelers, Peace Corps volunteers, State Department employees, and military personnel are increasingly at risk, and our ability to protect and cure them is in jeopardy. What is desperately needed is a better application of existing malaria control tools and new methods of containing the disease.
In most malarious regions of the world, there is inadequate access to malaria treatment. Appropriate health facilities may not exist; those that do exist may be inaccessible to affected populations, may not be supplied with effective drugs, or may be staffed inappropriately. In many countries, the expansion of primary health care services has not proceeded according to expectations, particularly in the poorest (and most malarious) nations of the tropical world.
In some countries, antimalarial interventions are applied in broad swaths, without regard to underlying differences in the epidemiology of the disease. In other countries, there are no organized interventions at all. The malaria problem in many regions is compounded by migration, civil unrest, poorly planned exploitation of natural resources, and their frequent correlate, poverty.
During the past 15 years, much research has focused on developing vaccines for malaria. Malaria vaccines are thought to be possible in part because people who are naturally exposed to the malaria parasite acquire a partial immunity to the disease over time. In addition, immunization of animals and humans by the bites of irradiated mosquitoes infected with the malaria parasite can protect against malaria infection. Much progress has been made, but current data suggest that effective vaccines are not likely to be available for some time.
Compounding the difficulty of developing more effective malaria prevention, treatment, and control strategies is a worldwide decline in the pool of scientists and health professionals capable of conducting field research and organizing and managing malaria control programs at the country level. With the change in approach from malaria eradication to malaria control, many malaria programs “lost face,” admitting failure and losing the priority interest of their respective ministries of health. As external funding agencies lost interest in programs, they reduced their technical and financial support. As a consequence, there were fewer training opportunities, decreased contacts with international experts, and diminished prospects for improving the situation. Today, many young scientists and public health specialists, in both the developed and developing countries, prefer to seek higher-profile activities with better defined opportunities for career advancement.
It is against this backdrop of a worsening worldwide malaria situation that the Institute of Medicine was asked to convene a multidisciplinary committee to assess the current status of malaria research and control and to make recommendations to the U.S. government on promising and feasible strategies to address the problem. During the 18-month study, the committee reviewed the state of the science in the major areas of malariology, identified gaps in knowledge within each of the major disciplines, and developed recommendations for future action in malaria research and control.
Organization
Chapter 2 summarizes key aspects of the individual state-of-the-science chapters, and is intended to serve as a basic introduction to the medical and scientific aspects of malaria, including its clinical signs, diagnosis, treatment, and control. Chapter 3 provides a historical overview of malaria, from roughly 3000 B.C. to the present, with special emphasis on efforts in this century to eradicate and control the disease. The state-of-the-science reviews, which start in Chapter 4 , begin with a scenario titled “Where We Want To Be in the Year 2010.” Each scenario describes where the discipline would like to be in 20 years and how, given an ideal world, the discipline would have contributed to malaria control efforts. The middle section of each chapter contains a critical review of the current status of knowledge in the particular field. The final section lays out specific directions for future research based on a clear identification of the major gaps in scientific understanding for that discipline. The committee urges those agencies that fund malaria research to consult the end of each state-of-the-science chapter for suggestions on specific research opportunities in malaria.
Sponsorship
This study was sponsored by the U.S. Agency for International Development, the U.S. Army Medical Research and Development Command, and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
- CONCLUSIONS AND RECOMMENDATIONS
A major finding of the committee is the need to increase donor and public awareness of the growing risk presented by the resurgence of malaria. Overall, funding levels are not adequate to meet the problem. The committee believes that funding in the past focused too sharply on specific technologies and particular control strategies (e.g., indiscriminate use of insecticide spraying). Future support must be balanced among the needs outlined in this report. The issue for prioritization is not whether to select specific technologies or control strategies, but to raise the priority for solving the problem of malaria. This is best done by encouraging balanced research and control strategies and developing a mechanism for periodically adjusting support for promising approaches.
This report highlights those areas which the committee believes deserve the highest priority for research or which should be considered when U.S. support is provided to malaria control programs. These observations and suggestions for future action, presented below in four sections discussing policy, research, control, and training, represent the views of a multidisciplinary group of professionals from diverse backgrounds and with a variety of perspectives on the problem.
The U.S. government is the largest single source of funds for malaria research and control activities in the world. This investment is justified by the magnitude of the malaria problem, from both a foreign aid and a public health perspective. The increasing severity of the threat of malaria to residents of endemic regions, travelers, and military personnel, and our diminishing ability to counter it, should be addressed by a more comprehensive and better integrated approach to malaria research and control. However, overall U.S. support for malaria research and control has declined over the past five years. The committee believes that the amount of funding currently directed to malaria research and control activities is inadequate to address the problem.
Over the past 10 years, the majority of U.S. funds available for malaria research have been devoted to studies on immunity and vaccine development. Although the promise of vaccines remains to be realized, the committee believes that the potential benefits are enormous. At the same time, the relative paucity of funds available for research has prevented or slowed progress in other areas. Our incomplete knowledge about the basic biology of malaria parasites, how they interact with their mosquito and human hosts, and how human biology and behavior affect malaria transmission and control remains a serious impediment to the development and implementation of malaria control strategies. The committee believes that this situation must be addressed without reducing commitment to current research initiatives. The committee further believes that such research will pay long-term dividends in the better application of existing tools and the development of new drugs, vaccines, and methods for vector control.
The committee recommends that increased funds be made available so that U.S. research on malaria can be broadened according to the priorities addressed in this report, including laboratory and field research on the biology of malaria parasites, their mosquito vectors, and their interaction with humans.
The committee believes that the maximum return on investment of funds devoted to malaria research and control can be achieved only by rigorous review of project proposals. The committee further believes that the highest-quality review is essential to ensure that funding agencies spend their money wisely. The committee believes that all U.S.-supported malaria field activities, both research and control, should be of the highest scientific quality and relevance to the goals of malaria control.
The committee recommends decisions on funding of malaria research be based on scientific merit as determined by rigorous peer review, consistent with the guidelines of the National Institutes of Health or the United Nations Development Program/World Bank/ World Health Organization Special Programme for Research and Training in Tropical Diseases, and that all U.S.-supported malaria field projects be subject to similar rigorous review to ensure that projects are epidemiologically and scientifically sound.
Commitment and Sustainability
For malaria control, short-term interventions can be expected to produce only short-term results. The committee believes that short-term interventions are justified only for emergency situations. Longer-term interventions should be undertaken only when there is a national commitment to support sustained malaria surveillance and control.
The committee recommends that malaria control programs receive sustained international and local support, oriented toward the development of human resources, the improvement of management skills, the provision of supplies, and the integration of an operational research capability in support of an epidemiologically sound approach to malaria control.
Surveillance
During the major effort to eradicate malaria from many parts of the world that began in the late 1950s and ended in 1969, it was important to establish mechanisms to detect all malaria infections. As a result, systems were established in many countries to collect blood samples for later microscopic examination for the presence of parasites. Each year, the results from more than 140 million slides are reported to the World Health Organization, of which roughly 3 to 5 percent are positive for malaria. This approach seeks to answer the question posed 30 years ago: How many people are infected with the malaria parasite? It does not answer today's questions: Who is sick? Where? Why? The committee concludes that the mass collection of blood slides requires considerable resources, poses serious biosafety hazards, deflects attention from the treatment of ill individuals, and has little practical relevance for malaria control efforts today.
Instead of the mass collection of slides, the committee believes that the most effective surveillance networks are those that concurrently measure disease in human populations, antimalarial drug use, patterns of drug resistance, and the intensity of malaria transmission by vector populations. The committee believes that malaria surveillance practices have not received adequate recognition as an epidemiologic tool for designing, implementing, and evaluating malaria control programs.
The committee recommends that countries be given support to orient malaria surveillance away from the mass collection and screening of blood slides toward the collection and analysis of epidemiologically relevant information that can be used to monitor the current situation on an ongoing basis, to identify high-risk groups, and to detect potential epidemics early in their course.
Inter-Sectoral Cooperation
The committee believes that insufficient attention has been paid to the impact that activities in non-health-related sectors, such as construction, industry, irrigation, and agriculture, have on malaria transmission. Conversely, there are few assessments of the impact of malaria control projects on other public health initiatives, the environment, and the socioeconomic status of affected populations. Malaria transmission frequently occurs in areas where private and multinational businesses and corporations (e.g., hotel chains, mining operations, and industrial plants) have strong economic interests. Unfortunately and irresponsibly, some local and multinational businesses contribute few if any resources to malaria control in areas in which they operate.
The committee recommends greater cooperation and consultation between health and nonhealth sectors in the planning and implementation of major development projects and malaria activities. It also recommends that all proposed malaria control programs be analyzed for their potential impact on other public health programs, the environment, and social and economic welfare, and that local and multinational businesses be recruited by malaria control organizations to contribute substantially to local malaria control efforts.
New Tools for Malaria Control
The committee believes that, as a policy directive, it is important to support research activities to develop new tools for malaria control. The greatest momentum for the development of new tools exists in vaccine and drug development, and the committee believes it essential that this momentum be maintained. The committee recognizes that commendable progress has been made in defining the characteristics of antigens and delivery systems needed for effective vaccines, but that the candidates so far tested fall short of the goal. Much has been learned which supports the hope that useful vaccines can be developed. To diminish activity in vaccine development at this stage would deal a severe blow to one of our best chances for a technological breakthrough in malaria control.
The committee recommends that vaccine development continue to be a priority of U.S.-funded malaria research.
Only a handful of drugs are available to prevent or treat malaria, and the spread of drug-resistant strains of the malaria parasite threatens to reduce further the limited pool of effective drugs. The committee recognizes that there is little economic incentive for U.S. pharmaceutical companies to undertake antimalarial drug discovery activities. The committee is concerned that U.S. government support of these activities, based almost entirely at the Walter Reed Army Institute of Research (WRAIR), has decreased and is threatened with further funding cuts. The committee concludes that the WRAIR program in antimalarial drug discovery, which is the largest and most successful in the world, is crucial to international efforts to develop new drugs for malaria. The benefits of this program in terms of worldwide prevention and treatment of malaria have been incalculable.
The committee strongly recommends that drug discovery and development activities at WRAIR receive increased and sustained support.
The next recommendation on policy directions reflects the committee's concern about the lack of involvement in malaria research by the private sector. The committee believes that the production of candidate malaria vaccines and antimalarial drugs for clinical trials has been hampered by a lack of industry involvement. Greater cooperation and a clarification of the contractual relationships between the public and private sectors would greatly enhance the development of drugs and vaccines.
The committee recommends that mechanisms be established to promote the involvement of pharmaceutical and biotechnology firms in the development of malaria vaccines, antimalarial drugs, and new tools for vector control.
Coordination and Integration
The committee is concerned that there is inadequate joint planning and coordination among U.S.-based agencies that support malaria research and control activities. Four government agencies and many nongovernmental organizations in the United States are actively involved in malaria-related activities. There are also numerous overseas organizations, governmental and nongovernmental, that actively support such activities worldwide.
The complexity and variability of malaria, the actual and potential scientific advances in several areas of malariology, and most important the worsening worldwide situation argue strongly for an ongoing mechanism to assess and influence current and future U.S. efforts in malaria research and control.
The committee strongly recommends the establishment of a national advisory body on malaria.
In addition to fulfilling a much needed coordinating function among U.S.-based agencies and between the U.S. and international efforts, the national advisory body could monitor the status of U.S. involvement in malaria research and control, assess the relevant application of knowledge, identify areas requiring further research, make recommendations to the major funding agencies, and provide a resource for legislators and others interested in scientific policy related to malaria. The national advisory body could convene specific task-oriented scientific working groups to review research and control activities and to make recommendations, when appropriate, for changes in priorities and new initiatives.
The committee believes that the national advisory body should be part of, and appointed by, a neutral and nationally respected scientific body and that it should actively encourage the participation of governmental and nongovernmental organizations, industry, and university scientists in advising on the direction of U.S. involvement in malaria research and control.
The increasing magnitude of the malaria problem during the past decade and the unpredictability of changes in human, parasite, and vector determinants of transmission and disease point strongly to the need for such a national advisory body, which can be responsive to rapidly changing problems, and advances in scientific research, relating to global efforts to control malaria.
Malaria Research Priorities
Malaria control is in crisis in many areas of the world. People are contracting and dying of severe malaria in unprecedented numbers. To address these problems, the committee strongly encourages a balanced research agenda. Two basic areas of research require high priority. Research that will lead to improved delivery of existing interventions for malaria, and the development of new tools for the control of malaria.
Research in Support of Available Control Measures
Risk Factors for Severe Malaria People who develop severe and complicated malaria lack adequate immunity, and many die from the disease. Groups at greatest risk include young children and pregnant women in malaria endemic regions; nonimmune migrants, laborers, and visitors to endemic regions; and residents of regions where malaria has been recently reintroduced. For reasons that are largely unknown, not all individuals within these groups appear to be at equal risk for severe disease. The committee believes that the determinants of severe disease, including risk factors associated with a population, the individual (biologic, immunologic, socioeconomic, and behavioral), the parasite, or exposure to mosquitoes, are likely to vary considerably in different areas.
The committee recommends that epidemiologic studies on the risk factors for severe and complicated malaria be supported.
Pathogenesis of Severe and Complicated Malaria Even with optimal care, 20 to 30 percent of children and adults with the most severe form of malaria—primarily cerebral malaria—die. The committee believes that a better understanding of the disease process will lead to improvements in preventing and treating severe forms of malaria. The committee further believes that determining the indications for treatment of severe malarial anemia is of special urgency given the risk of transmitting the AIDS virus through blood transfusions, the only currently available treatment for malarial anemia. Physicians need to know when it is appropriate to transfuse malaria patients.
The committee recommends greater support for research on the pathogenesis of severe and complicated malaria, on the mechanisms of malarial anemia, and on the development of specific criteria for blood transfusions in malaria.
Social Science Research The impact of drugs to control disease or programs to reduce human-mosquito contact is mediated by local practices and beliefs about malaria and its treatment. Most people in malaria- endemic countries seek initial treatment for malaria outside of the formal health sector. Programs that attempt to influence this behavior must understand that current practices satisfy, at some level, local concerns regarding such matters as access to and effectiveness of therapy, and cost. These concerns may lead to practices at odds with current medical practice. Further, many malaria control programs have not considered the social, cultural, and behavioral dimensions of malaria, thereby limiting the effectiveness of measures undertaken. The committee recognizes that control programs often fail to incorporate household or community concerns and resources into program design. In most countries, little is known about how the demand for and utilization of health services is influenced by such things as user fees, location of health clinics, and the existence and quality of referral services. The committee concludes that modern social science techniques have not been effectively applied to the design, implementation, and evaluation of malaria control programs.
The committee recommends that research be conducted on local perceptions of malaria as an illness, health-seeking behaviors (including the demand for health care services), and behaviors that affect malaria transmission, and that the results of this research be included in community-based malaria control interventions that promote the involvement of communities and their organizations in control efforts.
Innovative Approaches to Malaria Control Malaria control programs will require new ideas and approaches, and new malaria control strategies need to be developed and tested. There is also a need for consistent support of innovative combinations of control technologies and for the transfer of new technologies from the laboratory to the clinic and field for expeditious evaluation. Successful technology transfer requires the exchange of scientific research, but more importantly, must be prefaced by an improved understanding of the optimal means to deliver the technology to the people in need (see Chapter 11 ).
The committee recommends that donor agencies provide support for research on new or improved control strategies and into how new tools and technologies can be better implemented and integrated into on-going control efforts.
Development of New Tools
Antimalarial Immunity and Vaccine Development Many people are able to mount an effective immune response that can significantly mitigate symptoms of malaria and prevent death. The committee believes that the development of effective malaria vaccines is feasible, and that the potential benefits of such vaccines are enormous. Several different types of malaria vaccines need to be developed: vaccines to prevent infection (of particular use for tourists and other nonimmune visitors to endemic countries), prevent the progression of infection to disease (for partially immune residents living in endemic areas and for nonimmune visitors), and interrupt transmission of parasites by vector populations (to reduce the risk of new infections in humans). The committee believes that each of these directions should be pursued.
The committee recommends sustained support for research to identify mechanisms and targets of protective immunity and to exploit the use of novel scientific technologies to construct vaccines that induce immunity against all relevant stages of the parasite life cycle.
Drug Discovery and Development Few drugs are available to prevent or treat malaria, and the spread of drug-resistant strains of malaria parasites is steadily reducing the limited pool of effective chemotherapeutic agents. The committee believes that an inadequate understanding of parasite biochemistry and biology impedes the process of drug discovery and slows studies on the mechanisms of drug resistance.
The committee recommends increased emphasis on screening compounds to identify new classes of potential antimalarial drugs, identifying and characterizing vulnerable targets within the parasite, understanding the mechanisms of drug resistance, and identifying and developing agents that can restore the therapeutic efficacy of currently available drugs.
Vector Control Malaria is transmitted to humans by the bites of infective mosquitoes. The objective of vector control is to reduce the contact between humans and infected mosquitoes. The committee believes that developments are needed in the areas of personal protection, environmental management, pesticide use and application, and biologic control, as well as in the largely unexplored areas of immunologic and genetic approaches for decreasing parasite transmission by vectors.
The committee recommends increased support for research on vector control that focuses on the development and field testing of methods for interrupting parasite transmission by vectors.
Malaria Control
Malaria is a complex disease that, even under the most optimistic scenario, will continue to be a major health threat for decades. The extent to which malaria affects human health depends on a large number of epidemiologic and ecologic factors. Depending on the particular combination of these and other variables, malaria may have different effects on neighboring villages and people living in a single village. All malaria control programs need to be designed with a view toward effectiveness and sustainability, taking into account the local perceptions, the availability of human and financial resources, and the multiple needs of the communities at risk. If community support for health sector initiatives is to be guaranteed, the public needs to know much more about malaria, its risks for epidemics and severe disease, and difficulties in control.
Unfortunately, there is no “magic bullet” solution to the deteriorating worldwide malaria situation, and no single malaria control strategy will be applicable in all regions or epidemiologic situations. Given the limited available financial and human resources and a dwindling pool of effective antimalarial tools, the committee suggests that donor agencies support four priority areas for malaria control in endemic countries.
The committee believes that the first and most basic priority in malaria control is to prevent infected individuals from becoming severely ill and dying. Reducing the incidence of severe morbidity and malaria-related mortality requires a two-pronged approach. First, diagnostic, treatment, and referral capabilities, including the provision of microscopes, training of technicians and other health providers, and drug supply, must be enhanced. Second, the committee believes that many malaria-related deaths could be averted if individuals and caretakers of young children knew when and how to seek appropriate treatment and if drug vendors, pharmacists, physicians, nurses, and other health care providers were provided with up-to-date and locally appropriate treatment and referral guidelines. The development and implementation of an efficient information system that provides rapid feedback to the originating clinic and area is key to monitoring the situation and preventing epidemics.
The committee believes that the second priority should be to promote personal protection measures (e.g., bednets, screens, and mosquito coils) to reduce or eliminate human-mosquito contact and thus to reduce the risk of infection for individuals living in endemic areas. At the present time, insecticide-treated bednets appear to be the most promising personal protection method.
In many environments, in addition to the treatment of individuals and use of personal protection measures, community-wide vector control is feasible. In such situations, the committee believes that the third priority should be low-cost vector control measures designed to reduce the prevalence of infective mosquitoes in the environment, thus reducing the transmission of malaria to populations. These measures include source reduction (e.g., draining or filling in small bodies of water where mosquito larvae develop) or the application of low-cost larval control measures. In certain environments, the use of insecticide-impregnated bednets by all or most members of a community may also reduce malaria transmission, but this approach to community-based malaria control remains experimental.
The committee believes that the fourth priority for malaria control should be higher cost vector control measures such as large-scale source reduction or widespread spraying of residual insecticides. In certain epidemiologic situations, the use of insecticides for adult mosquito control is appropriate and represents the method of choice for decreasing malaria transmission and preventing epidemics (see Chapter 7 and Chapter 10 ).
The committee recommends that support of malaria control programs include resources to improve local capacities to conduct prompt diagnosis, including both training and equipment, and to ensure the availability of antimalarial drugs.
The committee recommends that resources be allocated to develop and disseminate malaria treatment guidelines for physicians, drug vendors, pharmacists, village health workers, and other health care personnel in endemic and non-endemic countries. The guidelines should be based, where appropriate, on the results of local operational research and should include information on the management of severe and complicated disease. The guidelines should be consistent and compatible among international agencies involved in the control of malaria.
The committee recommends that support for malaria control initiatives include funds to develop and implement locally relevant communication programs that provide information about how to prevent and treat malaria appropriately (including when and how to seek treatment) and that foster a dialogue about prevention and control.
Organization of Malaria Control
One of the major criticisms of malaria control programs during the past 10 to 15 years has been that funds have been spent inappropriately without an integrated plan and without formal evaluation of the efficacy of control measures instituted. In many instances, this has led to diminished efforts to control malaria.
The committee strongly encourages renewed commitment by donor agencies to support national control programs in malaria- endemic countries.
The committee recommends that U.S. donor agencies develop, with the advice of the national advisory body, a core of expertise (either in-house or through an external advisory group) to plan assistance to malaria control activities in endemic countries.
The committee believes that the development, implementation, and evaluation of such programs must follow a rigorous set of guidelines. These guidelines should include the following steps:
Identification of the problem
Determine the extent and variety of malaria. The paradigm approach described in Chapter 10 should facilitate this step.
Analyze current efforts to solve malaria problems.
Identify and characterize available in-country resources and capabilities.
Development of a plan
Design and prioritize interventions based on the epidemiologic situation and the available resources.
Design a training program for decision makers, managers, and technical staff to support and sustain the interventions.
Define specific indicators of the success or failure of the interventions at specific time points.
Develop a specific plan for reporting on the outcomes of interventions.
Develop a process for adjusting the program in response to successes and/or failures of interventions.
Review of the comprehensive plan by a donor agency review board
Modification of the plan based on comments of the review board
Implementation of the program
Yearly report and analysis of outcome variables
To guide the implementation of the activities outlined above, the committee has provided specific advice on several components, including an approach to evaluating malaria problems and designing control strategies (the paradigm approach), program management, monitoring and evaluation, and operational research.
Paradigm Approach
Given the complex and variable nature of malaria, the committee believes that the epidemiologic paradigms (see Chapter 10 ), developed in conjunction with this study, may form the basis of a logical and reasoned approach for defining the malaria problems and improving the design and management of malaria control programs.
The committee recommends that the paradigm approach be field tested to determine its use in helping policymakers and malaria program managers design and implement epidemiologically appropriate and cost-effective control initiatives.
The committee recognizes that various factors, including the local ecology, the dynamics of mosquito transmission of malaria parasites, genetically determined resistance to malaria infection, and patterns of drug use, affect patterns of malaria endemicity in human populations and need to be considered when malaria control strategies are developed. In most endemic countries, efforts to understand malaria transmission through field studies of vector populations are either nonexistent or so limited in scope that they have minimal impact on subsequent malaria control efforts. The committee recognizes that current approaches to malaria control are clearly inadequate. The committee believes, however, that malaria control strategies are sometimes applied inappropriately, with little regard to the underlying differences in the epidemiology of the disease.
The committee recommends that support for malaria control programs include funds to permit a reassessment and optimization of antimalarial tools based on relevant analyses of local epidemiologic, parasitologic, entomologic, socioeconomic, and behavioral determinants of malaria and the costs of malaria control.
Poor management has contributed to the failure of many malaria control programs. Among the reasons are a chronic shortage of trained managers who can think innovatively about health care delivery and who can plan, implement, supervise, and evaluate malaria control programs. Lack of incentives, the absence of career advancement options, and designation of responsibility without authority often hinder the effectiveness of the small cadre of professional managers that does exist. The committee recognizes that management technology is a valuable resource that has yet to be effectively introduced into the planning, implementation, and evaluation of most malaria control programs.
The committee recommends that funding agencies utilize management experts to develop a comprehensive series of recommendations and guidelines as to how basic management skills and technology can be introduced into the planning, implementation, and evaluation of malaria control programs.
The committee recommends that U.S. funding of each malaria control program include support for a senior manager who has responsibility for planning and coordinating malaria control activities. Where such an individual does not exist, a priority of the control effort should be to identify and support a qualified candidate. The manager should be supported actively by a multidisciplinary core group with expertise in epidemiology , entomology, the social sciences, clinical medicine, environmental issues, and vector control operations.
Monitoring and Evaluation
Monitoring and evaluation are essential components of any control program. For malaria control, it is not acceptable to continue pursuing a specific control strategy without clear evidence that it is effective and reaching established objectives.
The committee recommends that support for malaria control programs include funds to evaluate the impact of control efforts on the magnitude of the problem and that each program be modified as necessary on the basis of periodic assessments of its costs and effectiveness.
Problem Solving (Operational Research) and Evaluation
At the outset of any malaria prevention or control initiative and during the course of implementation, gaps in knowledge will be identified and problems will arise. These matters should be addressed through clearly defined, short-term, focused studies. Perhaps the most difficult aspects of operational research are to identify the relevant problem, formulate the appropriate question, and design a study to answer that question.
The committee recommends that a problem-solving (operational research) component be built into all existing and future U.S.-funded malaria control initiatives and that support be given to enhance the capacity to perform such research. This effort will include consistent support in the design of focused projects that can provide applicable results, analysis of data, and dissemination of conclusions.
The committee concludes that there is a need for additional scientists actively involved in malaria-related research in the United States and abroad. To meet this need, both short- and long-term training at the doctoral and postdoctoral levels must be provided. This training will be of little value unless there is adequate long-term research funding to support the career development of professionals in the field of malaria.
The committee recommends support for research training in malaria.
Whereas the curricula for advanced degree training in basic science research and epidemiology are fairly well defined, two areas require attention, especially in the developing world: social sciences and health management and training.
The committee recommends that support be given for the development of advanced-degree curricula in the social sciences, and in health management and training, for use in universities in developing and developed countries.
The availability of well-trained managers, decision makers, and technical staff is critical to the implementation of any malaria prevention and control program. The development of such key personnel requires a long term combination of formal training, focused short courses, and a gradual progression of expertise.
The committee recommends support for training in management, epidemiology , entomology, social sciences, and vector control. Such training for malaria control may be accomplished through U.S.-funded grant programs for long-term cooperative relationships between institutions in developed and developing countries; through the encouragement of both formal and informal linkages among malaria- endemic countries; through the use of existing training courses; and through the development of specific training courses.
The committee recommends further that malaria endemic countries be supported in the development of personnel programs that provide long-term career tracks for managers, decision makers, and technical staff, and that offer professional fulfillment, security, and competitive financial compensation.
- Cite this Page Institute of Medicine (US) Committee for the Study on Malaria Prevention and Control; Oaks SC Jr., Mitchell VS, Pearson GW, et al., editors. Malaria: Obstacles and Opportunities. Washington (DC): National Academies Press (US); 1991. 1, Conclusions and Recommendations.
- PDF version of this title (3.9M)
- Disable Glossary Links
In this Page
Recent activity.
- Conclusions and Recommendations - Malaria Conclusions and Recommendations - Malaria
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
The leading journal on malarial research, Malaria Journal serves the community interested in malaria in its broadest sense. ... Although Tanzania adopted and has been implementing effective interventions to control and eventually eliminate malaria, the disease is still a leading public health problem, and the country experiences hetero ...
P. falciparum is associated with severe malaria and complications in pregnancy ( Box 3 ); most malaria-related deaths are associated with this species, which kills ∼ 1,200 African children <5 ...
Malaria is a mosquito-borne disease that is caused by Plasmodium parasites. Patients with malaria experience flu-like symptoms and, in severe cases, the disease can progress to neurological ...
Malaria research toward disease elimination. Malaria is a life-threatening febrile illness caused by Plasmodium spp. parasites that are transmitted to humans by infected female Anopheles ...
This documentary video discusses the epidemiology of malaria; strategies for prevention, including vector control and vaccines; and the pipeline of promising new drugs for the fight to eliminate ma...
Aims and Scope. Malaria Journal is aimed at the scientific community interested in malaria in its broadest sense. It is the only journal that publishes exclusively articles on malaria and, as such, it aims to bring together knowledge from the different specialties involved in this very broad discipline, from the bench to the bedside and to the ...
Public health strategies for malaria in endemic countries aim to prevent transmission of the disease and control the vector. This historical analysis considers the strategies for vector control developed during the first four decades of the twentieth century. In 1925, policies and technological advances were debated internationally for the first time after the outbreak of malaria in Europe ...
Malaria is resurging in many African and South American countries, exacerbated by COVID-19-related health service disruption. In 2021, there were an estimated 247 million malaria cases and 619 000 deaths in 84 endemic countries. Plasmodium falciparum strains partly resistant to artemisinins are entrenched in the Greater Mekong region and have emerged in Africa, while Anopheles mosquito vectors ...
Malaria remains a disease of global health importance with 3.3 billion people in 97 countries at risk, leading to an estimated 200 million cases and around 600,000 deaths ... The authors acknowledge support from the National Health and Medical Research Council of Australia, PATH/MVI, USAID, and the Wellcome Trust. A.F.C. is an International ...
The need for further research to better ascertain the long term impact of MDA malaria campaigns has been identified ... Surveillance Atlas of Infectious Diseases. Malaria (accessed 4-1-23). ... •Malaria vaccine: WHO position paper - March 2022. Weekly Epidemiological Record,Vol. 97, No. 09, pp. 61-80. 4 March 2022. Geneva: World Health ...
As a result of the interplay between many factors, the control of this disease can be challenging. However, few studies have demonstrated malaria's complexity, control, and modeling although this perspective could lead to effective policy recommendations. This paper aims to be a didactic material providing the reader with an overview of malaria.
Read the latest Research articles in Malaria from Scientific Reports. ... a systematic review and meta-analysis of their association with disease severity. Saruda Kuraeiad, ... Calls for Papers
Malaria is a mosquito-borne infectious disease of humans and other animals caused by. protists (a type of microorganism) of the genus Plasmodium. It begins with a bite from an. infected female ...
Background Malaria is still a major global health burden, with more than 3.2 billion people in 91 countries remaining at risk of the disease. Accurately distinguishing malaria from other diseases, especially uncomplicated malaria (UM) from non-malarial infections (nMI), remains a challenge. Furthermore, the success of rapid diagnostic tests (RDTs) is threatened by Pfhrp2/3 deletions and ...
National Centre for Vector-Borne Diseases Control's (NCVBDC) malaria data (yearly) from district to the national level were used as a back-end data source for this dashboard. NCVBDC provided the data to the National Institute of Malaria Research upon request from the Indian Council of Medical Research (ICMR).
The research, published this week in Nature, tracks the disease over 5,500 years, with trade, warfare, colonialism, and slavery identified as major factors in its global spread. The findings represent a feat of collaboration and data-sharing, with 94 co-authors representing 80 institutions and 21 countries.
Great progress has been made in recent years to reduce the high level of suffering caused by malaria worldwide. Notably, the use of insecticide-treated mosquito nets for malaria prevention and the use of artemisinin-based combination therapy (ACT) for malaria treatment have made a significant impact. Nevertheless, the development of resistance to the past and present anti-malarial drugs ...
Background: Malaria is one of the infectious diseases of greatest interest to the scientific community and of greatest concern to international health authorities.Traditionally, the focus has been on Plasmodium falciparum, the parasite that causes the most severe form of the disease in Africa.However, in the last twenty years, the Plasmodium vivax parasite, responsible for a large number of ...
In 2022, approximately 12.7 million African women experienced pregnancy malaria 2. A 'disease of poverty', ... Ongoing research seeks to improve existing vaccines and to expand indications.
This paper addresses the above questions using a mathematical epidemiological model on COVID-19 and malaria co-dynamics. We used Nigeria as a case study in our analysis. Our choice for Nigeria was motivated by the heavy dependency of her populace on self-medication (see works in, for example, [13], [14], [15]), coupled with her having high ...
To produce the review, Hayes combed through the academic literature to find research on indoor pests like bed bugs, cockroaches and fleas, as well as papers on malaria, bed nets, pesticides and ...
Severe malaria is a medical emergency. It is a major cause of preventable childhood death in tropical countries. Severe malaria justifies considerable global investment in malaria control and elimination yet, increasingly, international agencies, funders and policy makers are unfamiliar with it, and so it is overlooked. In sub-Saharan Africa, severe malaria is overdiagnosed in clinical practice.
To produce the review, Hayes combed through the academic literature to find research on indoor pests like bed bugs, cockroaches and fleas, as well as papers on malaria, bed nets, pesticides and ...
Sub-Saharan Africa carried the brunt of the disease, where a child probably died of malaria every 45 seconds and efforts to control the disease were very limited. Yet this desperate situation had ...
One paper - a 2022 survey of 1,000 households in Botswana - found that while 58% were most concerned with mosquitoes in homes, more than 40% were most concerned with cockroaches and flies. Hayes said a recent paper - published after this NC State review was concluded - showed that people blamed the presence of bed bugs on bed nets.
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers, and 1.5 to 2.7 million people die in a year.[1] The Plasmodium parasite has a multistage lifecycle, which leads ...
Dr. Katie Young, lead author on the paper who undertook this research as a Ph.D. student in the Sharpe lab, said, "Through this work we aimed to investigate the role of PTPRK in the colon, working ...
Malaria is a life-threatening disease that involves the Plasmodium parasite, which poses a high death rate. ... microscopy and other existing methods for malaria diagnosis. This paper is ...
The outlook for malaria control is grim. The disease, caused by mosquito-borne parasites, is present in 102 countries and is responsible for over 100 million clinical cases and 1 to 2 million deaths each year. Over the past two decades, efforts to control malaria have met with less and less success. In many regions where malaria transmission had been almost eliminated, the disease has made a ...