Diffusion I: Random molecular movement and influences on diffusion rate
by Heather MacNeill Falconer, M.A./M.S., Gina Battaglia, Ph.D., Anthony Carpi, Ph.D.
Listen to this reading
Did you know that the process of diffusion is responsible for the way smells travel from the kitchen throughout the house? In diffusion, particles move randomly, beginning in an area of higher concentration and ending in an area of lower concentration. This principle is fundamental throughout science and is very important to how the human body and other living things function.
Diffusion is the process by which molecules move through a substance, seemingly down a concentration gradient, because of the random molecular motion and collision between particles.
Many factors influence the rate at which diffusion takes place, including the medium through with a substance is diffusing, the size of molecules diffusing, the temperature of the materials, and the distance molecules travel between collisions.
The diffusion coefficient, or diffusivity, provides a relative measure at specific conditions of the speed at which two substances will diffuse into one another.
If you’ve ever made cookies and left the kitchen door open, you’re probably aware that the aroma spreads throughout the house. It is strongest in the kitchen, where the cookies are baking, a little less in the dining or living room, and least in the upstairs corner bedroom. And if the door is closed in the corner bedroom, the cookie scent is even weaker.
This is a delicious example of diffusion , or the movement of matter from a region of high concentration (the cookie pan in the kitchen) to a region of low concentration (the corner bedroom). This principle of diffusion is fundamental throughout science, from gas exchange in the lungs to the spread of carbon dioxide in the atmosphere to the movement of water from one side of a cell’s plasma membrane to the other. However, the concept of diffusion is rarely as simple as molecules moving from one place to another. Temperature, the size of the molecules involved, the distance molecules need to travel, the barriers they may encounter along the way, and other factors all influence the rate at which diffusion takes place.
- Random walk: Molecular movement through a given space
The universe is in constant motion: from the orbiting of planets around the sun, to the movement of particles from one area to another. And while on a grand scale it may appear that there is a rationale to this movement – for example, the planets in our solar system have regular revolutions that can be predicted – in truth there is a great deal of motion that occurs randomly.
When we learn about diffusion , we often hear about the movement of particles from an area of high concentration to an area of low concentration, as if the particles themselves are somehow motivated to move in this direction. But this movement is in fact a by-product of what scientists refer to as the “random walk” of particles. Molecules do not move in straight paths from Point A to Point B. Instead, they interact with their environment , bumping into other molecules and barriers encountered along their way, as well as interacting with the medium through which they are moving.
The observation of the spontaneous, random movement of small particles was first recorded in the first century BCE . Lucretius, a Roman poet and philosopher, described the dust seen in sunbeams coming through a window (Figure 1):
You will see a multitude of tiny particles mingling in a multitude of ways... their dancing is an actual indication of underlying movements of matter that are hidden from our sight... It originates with the atoms which move of themselves [i.e., spontaneously]… So the movement mounts up from the atoms and gradually emerges to the level of our senses, so that those bodies are in motion that we see in sunbeams, moved by blows that remain invisible.

While Lucretius’s “dancing” particles were likely dust particles or pollen grains that are affected by air currents and other phenomenon, his description is a wonderfully accurate assessment of what goes on at the molecular level. Many scientists have explored this random molecular motion in a variety of contexts, most famously by the Scottish botanist Robert Brown in the 19 th century.
In 1828, while observing pollen granules suspended in water under a microscope, Brown discovered that the motion of the granules were “neither from currents in the fluid , nor from its gradual evaporation , but belonged to the particle itself.” After suspending various organic and inorganic substances in water and seeing this same inherent , random movement, he concluded that this random walk of particles – later termed Brownian motion in his honor – was a general property of matter that is suspended in a liquid medium. However, it would take nearly a century for scientists to mathematically quantify Brownian motion and demonstrate that this random movement of molecules dictates diffusion .

Comprehension Checkpoint
- What causes random molecular movement?
About the same time that Brown was making his observations , a group of scientists including the French engineer Sadi Carnot and German physicist Rudolph Clausius were establishing a whole new field of scientific study: the field of Thermodynamics (see our Thermodynamics I module for more information). Clausius’s work in particular led to the development of the kinetic theory of heat – the idea that atoms and molecules are in motion and the speed of that motion is related to a number of things, including the heat of the substance. The molecules of a solid are generally considered to be locked in place (though they vibrate); however, the molecules of a liquid or a gas are free to move around, and they do: bumping in to one another or the walls of their container like balls on a pool table.
As molecules in a liquid or gas move through space, they bump into one another and follow random paths – moving in a straight line until something blocks their way and then bouncing off of that thing. This random molecular movement is constantly occurring and can be measured, giving a molecule’s mean free path – or, the average distance a particle moves between impacts with other particles.
It is this spontaneous and random motion that leads to diffusion . For example, as the scent molecules from baking cookies move into the air, they interact with air molecules – crashing into them and changing direction. Over time, these random processes will cause the scent molecules to disperse throughout the room. Diffusion is presented as a process in which a substance moves down a concentration gradient – from an area of high concentration to an area of low concentration. However, it is important to recognize that there is no directional force at play – the scent molecules are not pushed to the edge of the room because the concentration is lower there. It is the random movement of these molecules within the roomful of moving air molecules that causes them to evenly spread out throughout the entire space – bouncing off walls, moving through doors, and eventually moving through the whole house. In this way, it appears to move along a concentration gradient – from the kitchen oven to the most distant rooms of the house.
- How concentration gradients work
It may sound like a paradox – the movement of molecules are random, yet at the same time appear to occur along a gradient – but in practice, it’s actually quite logical. A simple illustration of this process can be seen using a glass of water and food coloring. When a drop of food coloring enters the water, the food coloring molecules are highly concentrated at the location where the dye molecules meet the water molecules, giving the water in that area a very dark color (Figure 2). The bottom of the glass initially has few or no food coloring molecules and so remains clear. As the food coloring molecules begin to interact with the water molecules, molecular collisions cause them to move randomly around the glass. As collisions continue, the molecules spread out, or diffuse , over space.
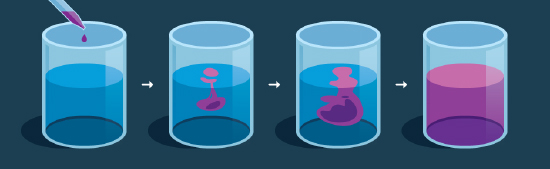
Figure 2 : Diffusion of a purple dye in a liquid.
Eventually, the molecules spread throughout the entire glass, becoming evenly distributed and filling the space. At this point, the molecules have reached a state of equilibrium in which no net diffusion is taking place and the concentration gradient no longer exists. In this state, the molecules are still moving haphazardly and colliding with each other; we just can’t see that motion because the water and color molecules are evenly dispersed throughout the space. Once equilibrium has been reached, the probability that a molecule will move from the top to the bottom is equal to the probability a molecule will move from the bottom to the top.
- Temperature and other factors influencing the rate of diffusion
We know that diffusion involves the movement of particles from one place to another; thus, the speed at which those particles move affects diffusion. Since molecular motion can be measured by the heat of an object, it follows that the hotter a substance is the faster diffusion will take place in that substance. (Click the animation below to see how temperature affects diffusion.) If you were to repeat your food coloring and water experiment comparing a glass of cold to a glass of hot water, you would see that the color disperses much more quickly in the hot water. But what other factors influence the speed, or rate, at which diffusion takes place?
Interactive Animation: The Effect of Temperature on Diffusion
- Size matters
In 1829, the Scottish physical chemist Thomas Graham first quantified diffusion behavior before the idea of atoms and molecules was widely established. Basing his observations on real-life “substances,” Graham measured the diffusion rates of gases through plaster plugs, fine tubes, and small orifices that were meant to slow down the diffusion process so that he could quantify it. One of his experiments , detailed in Figure 3, used an apparatus with the open end of a tube sitting in a beaker of water and the other end sealed with a plaster stopper containing holes large enough for gases to enter and leave the tube. Graham filled the open end of the tube with various gases (as indicated by the red tube in Figure 3), and observed the rate at which the gases effused , or escaped through the plaster plug. If the gas effused from the tube faster than the air outside of the tube moved in, the water level in the tube would rise. On the other hand, if the outside air moved through the plaster faster than the gas in the tube escaped to the outside, the water level in the tube would go down. He used the rate of change in the water level to determine the relative rate at which the different gases diffused into air.

Figure 3 : Thomas Graham's experiment to measure the diffusion rates of gases.
Graham experimented with many combinations of different gases and published his findings in an 1829 publication of the Quarterly Journal of Science, Literature, and Art titled “A Short Account of Experimental Researches on the Diffusion of Gases Through Each Other, and Their Separation by Mechanical Means.” He stated that when gases come into contact with each other, “indefinitely minute volumes” of the gases spontaneously intermix with each other until they reach equilibrium (Graham, 1829). However, he discovered that different types of gases did not mix at the same rate – rather, the rates at which two gases diffuse is inversely proportional to the square root of their densities, a relationship now known as Graham’s law . Although Graham’s original relationship used density , or mass per unit volume , the modern form of the equation uses molar mass, or the mass of one mole of a substance.
What Graham showed was that the molecular weight of a molecule directly affects the speed at which that molecule can move. Graham’s work actually helped lay the foundations of kinetic molecular theory because it recognized that at a given temperature, a heavy molecule would move more slowly than a light molecule. In other words, more kinetic energy is needed to move a large molecule at the same speed as a small molecule. You can think of it this way: A small push will get a tennis ball rolling quickly; however, it takes a much harder push to move a bowling ball at the same speed. At a given temperature, small molecules move faster, and will diffuse more quickly than large ones. View the animation below to see how atomic mass affects diffusion .
Interactive Animation: The Effect of Atomic Mass on Diffusion
- Solution properties
Graham later studied the diffusion of salts into liquids and discovered that the diffusion rate in liquids is several thousand times slower than in gases. This seems relatively obvious to us today, as we know that the molecules of a gas move faster and are more spread out than molecules in a liquid. Therefore, the movement of one substance within a gas occurs more freely than in a liquid. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. (View the animation below to compare diffusion in gases and liquids.) Diffusion, in fact, can even take place in solids . While this is a very slow process , Sir William Chandler Roberts-Austen, a British metallurgist, fused gold plates to the end of cylindrical rods made of lead. He analyzed the lead rods after a period of 31 days and actually found that gold atoms had “flowed” into the solid rods.
Interactive Animation: The Effect of State on Diffusion: Gases versus Liquids
- Concentration and the diffusion coefficient
While we have talked extensively about diffusion and concentration gradients, it was not until the mid-1800s when a German-born physicist and physiologist named Adolf Fick built upon Graham’s work and introduced the notion of a diffusion coefficient, or diffusivity, to characterize how fast molecules diffuse .
In his 1855 publication “On Diffusion” in Annalen der Physik , Fick described an experimental setup in which he connected cylindrical and conical tubes with solid salt crystals at the bottom to an “infinitely large” reservoir filled with freshwater (Figure 4). The solid salt crystals dissolved into the water in the tubes and diffused toward the water reservoir. A stream of freshwater swept the saltwater out of the reservoir. This stream of water kept the salt concentration at the very top of the tubes (the point where the salt solution met the water reservoir) close to zero. The dissolving salt at the bottom of the tube maintained a high salt concentration in the water at that end of the tube. Because the tubes had a different shape (conical versus cylindrical), the concentration gradient in the tubes differed, setting up a system in which diffusion could be compared in relation to a concentration gradient.
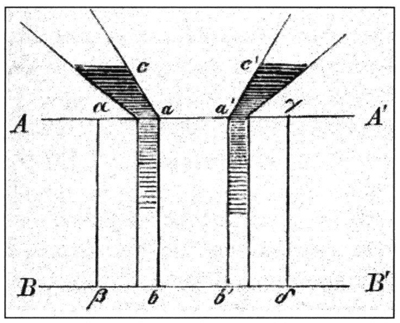
Figure 4 : Fick's experimental setup in which he connected cylindrical and conical tubes to a reservoir filled with freshwater. (Image from the 1903 publication, Collected Works, I . Stahel’sche Verlags-Anstalt, Würzburg: Germany.)
Fick then calculated the diffusion rate of the salt by measuring the amount of salt that passed through the top of the respective tubes (just before they met the freshwater in the reservoir) within a given time period. He discovered that the movement rate of the salt solution into the water reservoir depended on the concentration difference between the solution at the bottom of the tube and the concentration of the solution leaving the tube and entering the reservoir. In other words – the higher the concentration of salt at the top of the tube, the faster it diffused into the water reservoir. You can see how concentration affects diffusion in the animation below.
Interactive Animation: The Effect of Concentration on Diffusion
After studying the phenomenon, Fick hypothesized that the relationship between the concentration gradient and the diffusion rate was similar to what Joseph Fourier, a French mathematician and physicist, found in his study of heat conduction in 1822. Fourier had described the rate of heat transfer through a substance as proportional to the difference in temperature between two regions. Heat moves from warmer to cooler objects, and the greater the temperature difference between the two objects, the faster the heat moves. (This is why your mug of hot coffee cools off much faster outside on a cold morning than when you leave it in your heated apartment). Using Fourier’s law of thermal conduction as a model , Fick created a mathematical framework for the movement of salt into the water, proposing that the diffusion rate of a substance is proportional to the difference in concentration between the two regions. What this means for diffusion of a substance is that if the concentration of a given substance is high in relation to the substance it is diffusing into (e.g., food coloring into water), it will diffuse faster than if the concentration difference is low (e.g., food coloring into food coloring). The application of a successful principle from one branch of science to another is not uncommon, and Fick was a classic example of this process . Fick knew of Fourier’s work because he had modeled his experimental apparatus on that of Fourier. Thus it was natural for him to apply Fourier’s law to diffusion. While he had no way to know that the underlying mechanism of heat conduction and diffusion were both based on atomic collisions (in fact, some researchers at the time still doubted the existence of atoms), he had a feeling. That feeling, and the existence of atoms themselves, would be mathematically proven some 50 years later when Albert Einstein published his seminal work, Investigations on the Theory of the Brownian Movement (Einstein, 1905).
The diffusion coefficient, or diffusivity D , defined by Fick is a proportionality constant between the diffusion rate and the concentration gradient. The diffusion coefficient is defined for a specific solute-solvent pair, and the higher the value for the coefficient, the faster two substances will diffuse into one another. For example, at 25°C the diffusivity of gaseous air into gaseous water is 0.282 cm 2 /sec (Cussler, 1997). At the same temperature, the diffusivity of dissolved air into liquid water is 2.00 x 10 -5 cm 2 /sec, a much lower number than that for the two gases, representing the much slower diffusion rate in liquids compared to gases. And the diffusivity of dissolved helium into liquid water at 25°C is 6.28 x 10 -5 cm 2 /sec – higher than that of dissolved air, representing the smaller size of helium atoms compared to the nitrogen and oxygen molecules in air.
- Distance molecules travel
Yet another factor that influences the rate at which diffusion occurs is the distance a molecule travels before bumping into something (referred to as a molecule’s mean free path). Imagine taking a container filled with a gas and putting it under pressure so that the molecules in the gas are squeezed together. This would slow the rate of diffusion through the gas because the molecules travel a shorter distance before colliding with something else and changing direction. (The animation below shows the effect of pressure on diffusion.)
Interactive Animation: The Effect of Pressure on Diffusion
This is an important factor affecting the difference in diffusion rates in gases versus liquids versus solids ; because gas particles are the most spread out of the three, molecules travel the furthest between collisions and diffusion occurs most rapidly in this state (Figure 5).
Figure 5 : The three states of matter at the atomic level: gas, liquid, and solid.
To fully understand why we can smell the cookies baking in the kitchen from the bedroom we also have to consider another process at work here – advection . Advection involves the transfer of a material or heat due to the movement of a fluid . So, because people walk through the rooms of your house and because heat rises from your radiators, the air is constantly moving, and that movement carries and mixes the scent molecules in your house. In many situations (such as your house), the effects of advection exceed those of diffusion , but these processes work in tandem to bring you the cookie smell.
From the traveling smells of cookies to the dissolving of salt into water, diffusion is a process happening around (and within!) us every second of every day. It is a process that is critical to moving oxygen across the membranes of our lungs, moving nutrients through soil to be taken up by plants, dispersing pollutants that are released into the atmosphere , and a whole host of other events that are necessary for life to exist.
Table of Contents
Activate glossary term highlighting to easily identify key terms within the module. Once highlighted, you can click on these terms to view their definitions.
Activate NGSS annotations to easily identify NGSS standards within the module. Once highlighted, you can click on them to view these standards.
- Search Menu
Sign in through your institution
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Numismatics
- Classical Literature
- Classical Reception
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Epigraphy
- Greek and Roman Law
- Greek and Roman Archaeology
- Greek and Roman Papyrology
- Late Antiquity
- Religion in the Ancient World
- Social History
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Agriculture
- History of Education
- History of Emotions
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Acquisition
- Language Variation
- Language Families
- Language Evolution
- Language Reference
- Lexicography
- Linguistic Theories
- Linguistic Typology
- Linguistic Anthropology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies (Modernism)
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Religion
- Music and Culture
- Music and Media
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Meta-Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Science
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Gender
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Lifestyle, Home, and Garden
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Politics
- Law and Society
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Legal System - Costs and Funding
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Restitution
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Oncology
- Medical Toxicology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Clinical Neuroscience
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Medical Ethics
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Security
- Computer Games
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Neuroscience
- Cognitive Psychology
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Strategy
- Business History
- Business Ethics
- Business and Government
- Business and Technology
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Social Issues in Business and Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic Systems
- Economic Methodology
- Economic History
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Research Methodology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Management of Land and Natural Resources (Social Science)
- Natural Disasters (Environment)
- Pollution and Threats to the Environment (Social Science)
- Social Impact of Environmental Issues (Social Science)
- Sustainability
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- Ethnic Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Political Theory
- Political Behaviour
- Political Economy
- Political Institutions
- Politics and Law
- Politics and Religion
- Politics of Development
- Public Administration
- Public Policy
- Qualitative Political Methodology
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Disability Studies
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous chapter
- Next chapter >

3 3 Einstein's theory of diffusion
- Published: October 2012
- Cite Icon Cite
- Permissions Icon Permissions
Before it was universally accepted that a fluid consists of many moving molecules, Fick's Law and the diffusion equation were widely regarded as statements in continuum mechanics. With the molecular theory in mind, Einstein derived the diffusion equation from a model of random molecular motion instead of from a continuity equation and Fick's Law. This chapter presents Einstein's derivation and examine its strengths and weaknesses. The chapter then deduces some of the implications of Einstein's model of diffusion: its novel probabilistic perspective on the classical diffusion equation; its predictions for the mean of the square of the displacement of a single solute molecule in a given time, and the consequent single-molecule interpretation of the diffusion coefficient; its formulas for the covariance and the correlation of the displacement; its implied formula for the diffusion coefficient of one solute molecule relative to another; and its implied notion of single-molecule probability flux. Finally, The chapter takes the first step toward deriving a formula for the stochastic rate of a diffusion-controlled bimolecular chemical reaction, a derivation which will be will completed in Chapter 4.
Signed in as
Institutional accounts.
- Google Scholar Indexing
- GoogleCrawler [DO NOT DELETE]
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
- Add your ORCID iD
Institutional access
Sign in with a library card.
- Sign in with username/password
- Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.

Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

The fathers of the diffusion concept
Joseph Fourier (1768-1830) and Henri Navier (1785-1836), whose portraits are shown in the Figure, were the first scientists to understand that diffusion transport was proportional to the derivative of the quantity transported. Almost simultaneously, in 1822, they proposed to link this property to the molecular agitation of the fluid. In the case of the transport of chemical species, the law established in the article associated with this focus was proposed in 1855 by Adolf Fick , a German physiologist (1829-1901) and professor at the University of Marburg. Joseph Fourier was a French physicist, becoming rector and prefect of Grenoble, known for having proposed the analogous law for heat transfer in 1822, in his Théorie analytique de la chaleur , where he also introduced the trigonometric series now known as the Fourier series. Henri Navier was a civil engineering engineer who directed the construction of about ten bridges in France. At the Ecole Polytechnique, he was Joseph Fourier’s student.
In the case of viscous fluids subjected to shear, the law that expresses the friction between the various fluid layers is called Isaac Newton (1643-1727), an English scientist who made very important scientific advances. This is because Newton was the first to propose that the flow resistance of a fluid should be a linear function of its velocity, which is only true for slow flows. His book Principia Naturalis Principia Mathematica (1687) marked the history of science. The first theoretical explanation of the proportionality between the drag of the fluid when a small sphere falls and the velocity of this sphere is due to George Stokes (1819-1903) who draws it from the law published by Navier in 1822.
The fundamental equation of fluid mechanics is an extension of the initial equation proposed by Euler (1757) for a so-called “perfect” fluid, i. e. without viscosity. Completed by the expression of tangential stress due to viscosity, derived from the work of Navier and Stokes, it is now universally known as the Navier-Stokes equation.
Molecular agitation is not directly observable, but the effect of multiple molecular shocks on micrometric-sized particles visible under the microscope can be observed. These particles are animated by an irregular movement, called Brownian movement in homage to the botanist Robert Brown who discovered the effect in 1827 . The movement of these particles, much larger than molecules, is not described by Boltzmann’s theory, but by a theory proposed by Albert Einstein in 1905. This very general theory also applies to diffusion in a liquid (see also the article associated with this focus). Einstein thus indicated how to deduce the molecular dimensions of Brownian motion observations. This is what Jean Perrin did in 1909, thus obtaining the first determination of the number of Avogadro, and therefore of the mass of atoms. This provided an experimental confirmation of the existence of atoms, which was still hypothetical at the time.
Cover image. Portrait of Adolf Fick, chosen from those in the figure because some authors use the term “fickian diffusion” to describe the transport of chemical species by molecular agitation.

IMAGES
VIDEO
COMMENTS
Diffusion is the movement of molecules from an area in which they are high in concentration to an area in which they are low in concentration. Molecules move down a concentration gradient until they are equally distributed, or equilibrium is reached (Fig. 1).
The kinetic molecular theory (KMT) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. This theory is based on the following five postulates described here.
The module looks at historical developments in our understanding of diffusion, from observations of “dancing” particles in the first century BCE to the discovery of Brownian motion to more recent experiments. Topics include concentration gradients, the diffusion coefficient, and advection.
Our hypothesis about this relationship was that as the temperature of a given solution increases, the rate of diffusion through a semipermeable membrane will also increase, because the amount of thermal energy present in the solution is directly proportional to the rate of diffusion.
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential.
Diffusion • This “jiggling about” by lots of molecules leads to diffusion • Individual molecules follow a random walk, due to collisions with surrounding molecules • Diffusion = many random walks by many molecules – Substance goes from region of high concentration to region of lower concentration
To understand this process you need to understand the makeup of the cell membrane and an important phenomenon known as diffusion. Diffusion is the movement of a substance from an area of high concentration to an area of low concentration due to random molecular motion. All atoms and molecules possess kinetic energy, which is the energy of movement.
With the molecular theory in mind, Einstein derived the diffusion equation from a model of random molecular motion instead of from a continuity equation and Fick's Law. This chapter presents Einstein's derivation and examine its strengths and weaknesses.
Diffusion is defined as the net movement of molecules or ions from a region of high concentration to a region of lower concentration. Diffusion continues until a state of equilibrium is reached, which means that the molecules are randomly distributed throughout the system.
In the case of viscous fluids subjected to shear, the law that expresses the friction between the various fluid layers is called Isaac Newton (1643-1727), an English scientist who made very important scientific advances.