- Chemistry Articles
- Effect Of Concentration On The Rate Of Reaction Between Sodium Thiosulphate And Hydrochloric Acid

Effect of concentration on the rate of reaction between sodium thiosulphate and hydrochloric acid
In this article, we have discussed the effect of concentration on the rate of reaction between hydrochloric acid and sodium thiosulphate.
The aim of this experiment – Understanding the effect of concentration on the rate of reaction between hydrochloric acid and sodium thiosulphate.
The reaction between Sodium thiosulphate (Na 2 S 2 O 3 ) and hydrochloric acid (HCl)
To produce a colloidal solution of sulphur, where the solution obtained is translucent.
The reaction occurs as follows: Na 2 S 2 O 3 (aq) + 2HCl (aq) → 2NaCl (aq) + H 2 O(l) + SO 2 (g) + S(s)
The above reaction when written in its ionic form: S 2 O 3 -2 (aq) + 2H + (aq) → H 2 O (l) + SO 2 (g) + S(s)
As the temperature of the system increases or as the concentration of reacting species increases the rate of precipitation of sulphur also increases. As the concentration increases, molecular collisions per unit time of the reacting species increase which can result in increased chances of product formation. This results in an increase in the rate of precipitation of sulphur. Similarly, on increasing the temperature, the kinetic energy of the reacting species increases, so the number of collisions that result in the formation of products increase leading to a faster rate of reaction.
Materials required:
The apparatus and materials required for this experiment are as follows:
- Burette of volume 50 mL
- Burette stand
- Sodium thiosulphate
- 1M Hydrochloric acid
The effect of concentration on the rate of reaction:
- Take five conical flasks, rinse them with water, and label them 1, 2, 3, 4, 5.
- Add 10 mL of sodium thiosulphate solution in flask 1, 20 mL of sodium thiosulphate solution in flask 2, 30 mL of sodium thiosulphate solution in flask 3, 40 mL of sodium thiosulphate solution in flask 4, and 50 mL of sodium thiosulphate solution in flask 5.
- Add 40 mL of distilled water in the flask 1, 30 mL of distilled water in the flask 2, 20 mL of distilled water in flask 3, 10 mL of distilled water in flask 4. This is done to adjust the volume of solution in each flask to 50 mL.
- Add 1M HCl of volume 10 mL in flask 1 with the help of a burette.
- Start the stopwatch immediately.
- Take a white tile and draw a cross mark on it.
- Add half of the HCl in the flask 1 and shake it well and start the stop-watch.
- Observe the flask and start the stop-watch as soon as the cross mark becomes invisible. Record the time taken for the process.
- Repeat the experiment by adding 10 mL HCl in flask 2, 3, 4, 5 and record the time for each.
Observation and result
| Flask
| Sodium thiosulphate
| Distilled water volume
| HCl volume
| Time
|
| 1 | 10 mL | 40 mL | 10 | |
| 2 | 20 mL | 30 mL | 10 | |
| 3 | 30 mL | 20 mL | 10 | |
| 4 | 40 mL | 10 mL | 10 | |
| 5 | 50 mL | 0 mL | 10 |
Precautions to be taken during the experiment:
- Thoroughly wash the apparatus.
- The solutions taken for this experiment should be measured accurately.
- Use the same tile for all the observations.
- Stay alert while you start and stop the stop-watch.
1. What is the amount of sodium thiosulphate added in flask 1?
Ans: 10 mL.
2. What is the amount of distilled water added in flask 1?
Ans: 40 mL.
3. What is the amount of HCl added in all the flasks?
4. name the two solutions used for the experiment..
Ans: Sodium thiosulphate and hydrochloric acid.
5. How many conical flasks are required for this experiment?

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
Talk to our experts
1800-120-456-456
- Effect of Concentration on Rate of Reaction

Introduction
In a reaction, many elements or compounds react together to form one or more new products based on the basic elements of the compounds involved in the chemical reaction. The reaction may occur between solids, liquids, and gases, and the rate of each reaction is different from another which depends on a number of factors. Some chemical compounds called catalysts or enzymes help in accelerating the rate of reaction. This rate of reaction is also dependent on the amount or concentration of substances involved in the reaction.
In this article, the effect of concentration on the rate of the reaction between sodium thiosulphate and hydrochloric acid has been discussed in the form of an experiment.
About the Rate of Reaction
In any chemical reaction, several reactants react to form one or more new products. These reactants can be gases, solids or even liquids. The rate of reaction depends on many determinants or factors. The presence of catalysts can also accelerate the speed of any reaction. One of the essential components that determine the rate of reaction is the concentration of the reactants taking part in the chemical reaction. In this article, we are going to study the effect of concentration on reaction rate by observing a chemical reaction. The results obtained from the reaction between Na 2 S 2 O 3 and HCl helps you to understand the topic more clearly.
To understand the effect of concentration on the rate of reaction between sodium thiosulphate (Na 2 S 2 O 3 ) and hydrochloric acid (HCl).
The rate of the reaction directly depends on the products of the molar concentration of reactants. In this experiment, we will study the reaction between Sodium thiosulphate (Na 2 S 2 O 3 ) with hydrochloric acid (HCl).
Na 2 S 2 O 3 (aq) + 2HCL (aq) → H 2 O (l) + SO 2 (g)+ 2 NaCl (aq) + S (s)
We can also write the above reaction in ionic form as:
S 2 O 3 -2 (aq) + 2H + (aq) → H 2 O (l) + S (s) + SO 2 (g)
The solution obtained after the reaction is opaque and has a milky appearance due to the presence of sulphur. If we increase the temperature or concentration of the reactants, then the rate of precipitation of sulphur also increases. It happens because when concentration increases, molecular collisions also increase per unit time, which results in a fast rate of product formation.
The reaction is between the aqueous solutions of Na 2 S 2 O 3 and HCl, the rate of which directly depends on the product of the molar concentration of each component of the reaction.
The chemical reaction formula between HCl and Na 2 S 2 O 3 is as follows:
Here, we can see the products of the reaction are water , sulphur dioxide (SO 2 ), sodium chloride (NaCl) and a sulphur atom . Where SO 2 is formed in the gaseous state, NaCl is formed in the aqueous state and sulphur collects in the solid-state.
With the increase in the temperature of the system, the precipitation of sulphur increases. With the increase in concentration, a collision between molecules also increases which increases the chances of getting the products of the reaction, and an increase in temperature provides more kinetic energy to the reaction which in turn increases the rate of reaction, thereby, resulting in faster production of products.
Materials Required
The materials and apparatus required for conducting the reaction are as follows:
Five flasks of 100ml each
Two burettes of volume 50 ml each
Burette stand
Sodium thiosulphate
1M Hydrochloric acid
Five conical flasks (100 ml)
Two burettes
Burette Stand
Sodium Thiosulphate
1M Hydrochloric Acid Solution
First of all, take five conical flasks and rinse them with water to clean any residue. Now, label them as 1, 2, 3, 4 and 5 respectively.
Draw the cross mark on any white tile.
Take a burette and add 10 ml of Na 2 S 2 O 3 solution in flask 1 using it. Similarly, add 20 ml of Na 2 S 2 O 3 solution in flask 2, 30 ml in flask 3, 40 ml in flask 4 and 50 ml solution in flask 5.
Now, add distilled water in every flask such that the combined volume of water and Na 2 S 2 O 3 solution becomes 50 ml. It means we need to add 40ml, 30ml, 20 ml and 10 ml distilled water in flasks 1, 2, 3 and 4 respectively.
Take 10 ml of 1M HCl solution in a test tube using the burette. Add it in flask 1, which contains 40 ml water and 10 ml Na 2 S 2 O 3 . Shake it thoroughly and then start the stopwatch immediately.
Place the flask on the white tile having a cross mark. Observe the cross mark from the top and stop the stopwatch as the cross mark becomes invisible. Note the time taken for the whole process.
Repeat the same procedure with flask 2, 3, 4 and 5. Note the time when the cross mark becomes invisible in every container.
Wash the flasks and add 10ml of sodium thiosulphate in the first flask and add 10 ml more to each subsequent flask.
Add 40ml of distilled water in the first flask, 30ml in the second, 20ml in the third, 10ml in the fourth and none in the fifth flask.
10ml HCl is to be added to the first flask with the help of a burette and the stopwatch should be started immediately.
Take a white tile and put a cross mark on it distinctly. Put the first flask on the time and observe.
Observe till the solution is milky and the mark on the tile is visible and note the time right there.
Observations and Results
Flask Number | The volume of Sodium Thiosulphate present in the flask | The volume of Distilled water present in the flask | The volume of HCl added in the solution | Time |
1 | 10 ml | 40 ml | 10 ml | ……….s |
2 | 20 ml | 30 ml | 10 ml | ……….s |
3 | 30 ml | 20 ml | 10 ml | ……….s |
4 | 40 ml | 10 ml | 10 ml | ……….s |
5 | 50 ml | 0 ml | 10 ml | ……….s |
(Image will be uploaded soon)
The above image shows the graph between 1/t (on the y-axis) and the concentration of Na 2 S 2 O 3 (on the x-axis). We will obtain a straight sloping line, as shown in the figure.
From the above graph, it is clear that 1/t is directly proportional to the concentration of Na 2 S 2 O 3 solution taken during the experiment. We know that 1/t is the direct measure of the rate of reaction. Hence, the pace of chemical reaction in this case directly depends on the concentration of Na 2 S 2 O 3 . However, it doesn't mean that the speed of any chemical reaction doesn't depend on conc. of HCl. We will see a similar result if we keep the concentration of sodium thiosulphate constant and raise the concentration of hydrochloric acid. This experiment clearly shows the effect of concentration on Rate of Reaction.
The product formed from the reaction is milky in appearance due to the presence of sulphur. Increasing the temperature increases the rate of precipitation.
Noting the time and plotting a graph with 1/t on the y-axis and the concentration of Na 2 S 2 O 3 on the x-axis where t is the time taken to form products at different concentrations. It was observed that the graph shows a straight sloping line which means that 1/t is directly proportional to the concentration of Na 2 S 2 O 3 which means increasing the concentration increased the rate of reaction as well.
If the concentration of sodium thiosulphate was kept constant and the concentration of hydrochloric acid was increased, the same trend would be observed.

FAQs on Effect of Concentration on Rate of Reaction
1. What are the determining factors which affect the rate of reaction?
From the above experiment conducted, it is observed that the concentration of reactants has a direct effect on the rate of reaction. Other factors include temperature conditions given to the reaction, state of existence of the reactants, presence of catalysts and changes in the surface area affecting the rate of reaction.
2. What is the amount of HCl added to the flasks?
10 ml of HCl was added to each of the flasks.
To know more about the theory, materials required, observation, results, and conclusion of the experiment on the effect of concentration on the rate of reaction between sodium thiosulphate and hydrochloric acid, visit Vedantu's website or download the app. Vedantu gives you solutions to your queries as well as free resources to study from which you can download in PDF format and access anytime, anywhere!
3. What was the result of the reaction?
The result of the reaction can be summed up as follows:
It was observed that in a reaction, with an increase in the concentration of sodium thiosulphate gradually while keeping the concentration of hydrochloric acid constant, the rate of reaction has increased slowly. If “t” is the amount of time taken for the products to form then 1/t is directly proportional to the concentration of sodium thiosulphate.
The same result will be obtained if the concentration of hydrochloric acid was increased gradually while the concentration of sodium thiosulphate was kept constant.
4. What are the precautions to be taken during the experiment to study the effect of concentration on reaction rate?
First of all, one must ensure that the apparatus must be thoroughly clean before starting the process. Any impurities can lead to inaccuracy during the experiment. Moreover, it is essential to measure the volumes of the distilled water, sodium thiosulphate and hydrochloric acid accurately. You must use the same tile with the same cross mark for all the observations. The temperature variation can also affect the rate of reaction. Hence, it is essential to complete to prevent any temperature variation. Start the stopwatch immediately as you add the HCl in the solution. Finally, view the cross mark on the tile from the top from the same height during the observations.
5. What are the determinants that can affect the rate of reaction?
There is a direct effect of concentration on Rate of Reaction, as shown in the above experiment. The physical state of the reactants' surface area can also affect the pace of any reaction. For instance, if any metal reacts with gas, then only the molecules present at the surface of the metal can react with gas molecules. Hence, we can increase the surface area of reactants by cutting them in pieces to increase its rate of reaction. An increase in temperature can also enhance the rate of reaction because it will boost the kinetic energy of reactant particles. A presence of a catalyst can also accelerate the chemical reaction.
| Core Chemistry 14 - 16
Particles can only react if they collide - and they not only have to collide, but must collide with enough energy for something to happen, and may need to collide the right way round if the particle is a more complicated shape than a single atom or ion. Increasing the concentration simply means that the particles are going to hit each other more often.
A simple quick experiment Take the reaction between marble chips and dilute hydrochloric acid, for example. CaCO (s) + 2HCl(aq) (aq) + CO (g) + H O(l) If you do this with small marble chips and ordinary bench dilute hydrochloric acid, you get a good supply of bubbles of carbon dioxide produced. If you dilute the hydrochloric acid by a factor of 10, the reaction is much less vigorous and the flow of carbon dioxide is much slower. | |
| In fact, you will also only get a tenth of the volume of carbon dioxide produced if you collect it all. The marble chips are likely to be in large excess, and the amount of carbon dioxide produced is limited by the amount of acid. | |
| A more detailed experiment A commonly used experiment to show the effect of concentration on rate is between dilute hydrochloric acid and sodium thiosulfate solution. Na S O (aq) + 2HCl(aq) (g) + S(s) + H O(l) At this stage, the only place you are likely to come across sodium thiosulfate is in this reaction. The interesting thing about the reaction is the formation of a precipitate of sulfur. This is formed slowly and appears first as a very pale cream solid which turns yellow as more of it is formed. In the video you are going to watch, the time taken to form a very small fixed amount of sulfur is measured at various concentrations of sodium thiosulfate, keeping everything else the same. As you will see, the more dilute the sodium thiosulfate, the longer the time it takes for that amount of sulfur to form. The is the one that changes as a result of something you are doing. In this case, the dependent variable is the time taken for the cross to disappear, because that is changing as a result of you changing the concentration. The is the one that you are changing - in this case, the concentration. The independent variable is always plotted on the x-axis, and the dependent one on the y-axis. The video showed a graph of the results of time taken against concentration and looked like this.
As it stands, this isn't actually very helpful. All it shows is that as you increase the concentration, the time taken for the cross to disappear gets less. But you can see that just by looking at it. It would be much better if we could find a more precise relationship between the rate of the reaction and the concentration. If you have read the page about the effect of on rates of reaction, you will have read about "initial rate" experiments. This is another initial rate experiment. You are finding the time taken for a very small amount of sulfur to be produced at the very beginning of the reaction as you vary concentration. If you could do a complete plot of the mass of sulfur being formed against time, you would get a curve starting steeply, slowing down, and then stopping - exactly like the one you saw on the previous page. But at the very beginning of the reaction, the curve is almost a straight line. So if you consider plots of the very early parts of three reactions to produce a fixed mass of sulfur in this experiment, the graphs would look like this.
The initial rates would be m/t , m/t and m/t grams of sulfur per second. You don't know what m is of course - that would depend, amongst other things, on how thick your cross was, and how good your eyesight is. But it is always going to be the same in every experiment. What you can say is that the initial rate is proportional to 1/t - or inversely proportional to t, if you prefer. If it takes half as long for the cross to disappear, the rate is twice as fast; if it takes 4 times as long for the cross to disappear, the rate is only a quarter as fast. On a graph, we can use this by plotting 1/t as a measure of rate. It isn't an actual rate, but it allows you to compare rates.
Doing this shows that in this reaction, you have a straight line relationship between concentration and rate - rate is proportional to concentration.
| |

Rates of reaction
Required practical 5, core practicals.

Aims of Experiment
How does the concentration of an acid affect the rate of reaction?
In this experiment you will:
- react magnesium ribbon with different concentrations of hydrochloric acid
- measure the volume of gas produced for each concentration
- use your results to work out how the rate of reaction is affected by the concentration of the acid
How does the concentration of sodium thiosulphate affect the rate of reaction?
- react different concentrations of sodium thiosulfate with hydrochloric acid
- use a stop clock to time how long it takes for the mixture to become cloudy for each concentration
- use your results to work out how the rate of reaction changes as the concentration of the sodium thiosulfate changes
Risk Asessment
As a general rule, eye protection (goggles) must be worn for all practicals.
| hazard | possible harm | precaution |
|---|---|---|
| hydrochloric acid | skin and eye irritation | avoid contact with the skin |
| gases escaping from reaction | may damage skin and eyes | place cotton wool at opening of conical flask to minimse gas escape |
| hot sodium thiosulfate solution | burns to the skin | do not heat above 60°C |
| sulfur dioxide | irritation to the eyes and lungs, particularly to people with asthma | lab needs to be well ventilated |
This risk assessment is provided as an example only, and you must perform your own risk assessment before doing this experiment.
Each group will need:
magnesium strips hydrochloric acid (3 concentrations) 250 ml conical flask 100 ml gas syringe
water bath sodium thiosulfate 50 ml measuring cylinder stop clock or stopwatch 10 ml measuring cylinder
Experiment Set-up
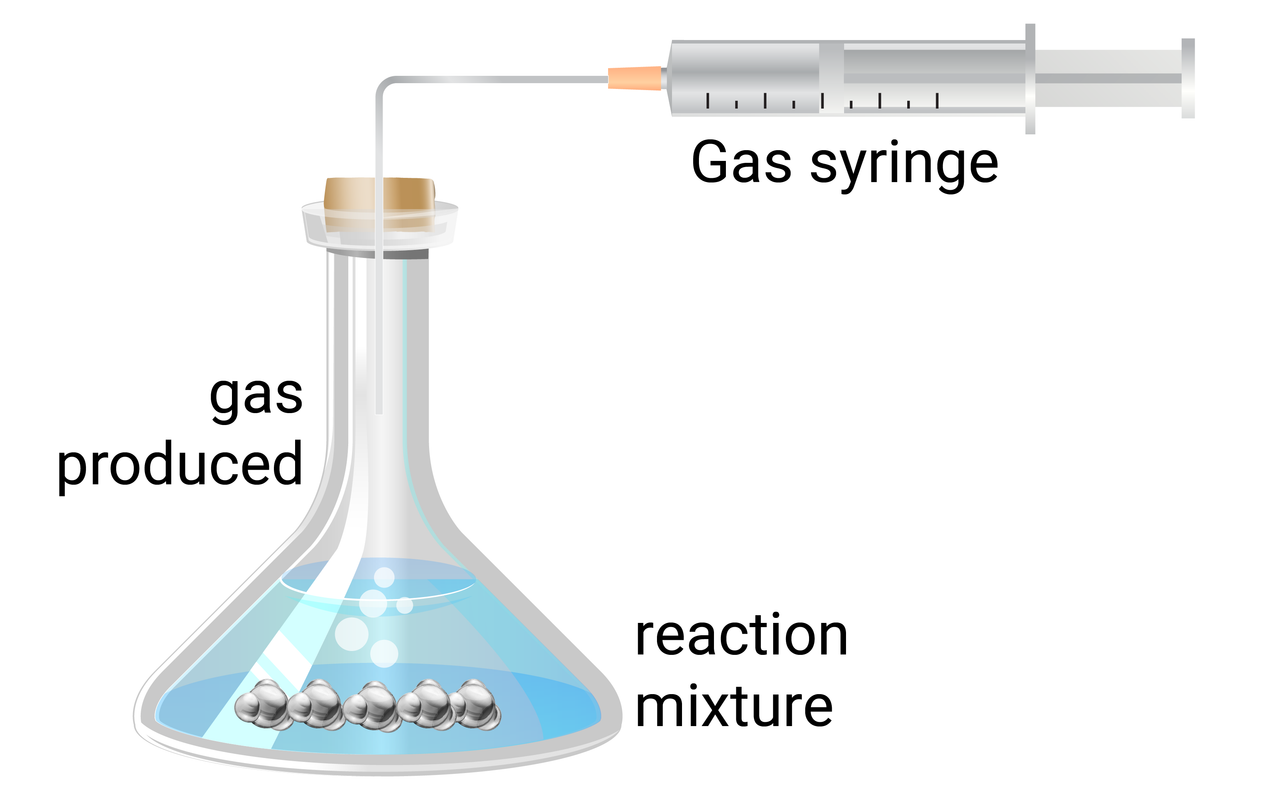
- use a measuring cylinder to add 50 ml of 0.5 mol/dm 3 hydrochloric acid to a conical flask
- add a single 3 cm strip of magnesium ribbon to the flask, and immediately connect the gas syringe and start a timer
- at every 20 seconds, record how much gas has been produced
- when the reaction is complete, clean the apparatus as instructed by your teacher
- repeat steps 1-4 with different concentrations (1.0 mol/dm 3 , and 1.5 mol/dm 3 ) of hydrochloric acid
- use a measuring cylinder to add 10 ml of sodium thiosulfate solution to a conical flask, then add 40 ml of water (concentration 8 g/dm 3 )
- measure and record the temperature of the solution
- place the conical flask on a piece of paper with a black cross drawn on it
- use another measuring cylinder to add 10 ml of hydrochloric acid to the flask, and immediately start a timer
- when the cross is no longer visible record the time taken, and then clean the apparatus as instructed by your teacher
- 20 ml sodium thiosulfate + 30 ml water (concentration 16 g/dm 3 )
- 30 ml sodium thiosulfate + 20 ml water (concentration 24 g/dm 3 )
- 40 ml sodium thiosulfate + 10 ml water (concentration 32 g/dm 3 )
- 50 ml sodium thiosulfate + no water (concentration 40 g/dm 3 ).
Results and Analysis
| time (s) | volume of gas produced (ml) | ||
|---|---|---|---|
| 0.5 mol/dm | 1.0 mol/dm | 1.5 mol/dm | |
| 0 | 0 | 0 | 0 |
| 20 | |||
| ... | |||
For each concentration plot a graph on the same set of axes to show:
- volume of gas (ml) on the Y axis (vertical)
- time (s) on the X axis (horizontal)
- a curve of best fit
Use your graph to compare the rates of reaction with different concentrations of hydrochloric acid with magnesium. Use collision theory to explain your findings.
| concentration (g/dm ) | time for cross to disappear (s) | ||
|---|---|---|---|
| trial 1 | trial 2 | mean | |
| 8 | |||
| 16 | |||
| ... | |||
Plot a graph to show:
- mean time (s) on the Y axis (vertical)
- concentration (g/dm 3 ) on the X axis (horizontal)
Describe the relationship between the independent variable and the dependent variable? What were your control variables? Evaluate the two methods that you have used to investigate the effect of concentration on rate of reaction.
Exam Question and Model Answer
A chemical company makes calcium chloride by reacting calcium carbonate and hydrochloric acid. They think they can increase the rate of reaction by increasing the concentration of the acid. Describe an experiment they could do in a laboratory to be able to test this idea.
Level 1 (1-2 marks)
Add calcium carbonate powder to a conical flask. Pour hydrochloric acid into the flask, mix, and immediately attach a gas syringe. Measure how much gas has been produced every 20 seconds, and record in a table. Compare the results to find out which reaction has a faster rate.
Level 2 (3-4 marks)
Add calcium carbonate powder to a conical flask. Pour hydrochloric acid into the flask, mix, and immediately attach a gas syringe. Measure how much gas has been produced every 20 seconds, and record in a table. Repeat these steps with other concentrations of hydrochloric acid as well (keep the volume the same). Compare the results to find out which reaction has a faster rate.
Level 3 (5-6 marks)
Add 5 g of calcium carbonate powder to a conical flask. Using a measuring cylinder, pour and mix 20 ml of 0.5 mol/dm 3 hydrochloric acid into the flask, and immediately attach a gas syringe. Measure how much gas has been produced every 20 seconds, and record in a table. Repeat these steps with 1.0 mol/dm 3 and 1.5 mol/dm 3 hydrochloric acid as well (keeping the temperature, volume of acid the same, and the mass of the calcium carbonate the same). Calculate the rate of each reaction (volume ÷ time), and compare the results to find out which reaction has a faster rate.

| P.O. Box 219 Batavia, IL 60510 | |
| 844-200-1455 | |
Rate of Reaction of Sodium Thiosulfate and Hydrochloric Acid
Publication 91860
Price: FREE
Learn more about downloading digital content
The purpose of this demonstration is to investigate the effect of sodium thiosulfate concentration on the rate of reaction of sodium thiosulfate with hydrochloric acid. The reaction, which produces solid sulfur, will be followed by measuring the time needed for the reaction mixture to become opaque. The results will be analyzed graphically to determine the order of reaction—the mathematical relationship between the reactant concentration and the rate.

- Ben Meacham's blog
Investigating the Effect of Concentration on Reaction Time

Whether you are introducing collision theory or something more demanding like reaction order, the reaction between sodium thiosulfate—Na 2 S 2 O 3 and hydrochloric acid can provide a consistent, accurate, and engaging opportunity for investigating these topics.
A few weeks ago, I was looking for a new reaction that could be used to investigate how concentration affects reaction time. In the past, I had always used traditional reactions such as magnesium and hydrochloric acid or Alka-Seltzer and hydrochloric acid. Though both served their purpose, there would always be groups that didn’t quite get data that was consistent with what I knew the relationship to be. In most cases, this was due to ambiguous and inconsistent timing methods or simply a matter of experimental error like not ensuring the magnesium stayed in the acid without floating to the top. I wanted a reaction that would be more likely to produce consistent results from group to group, easy to execute, and was a bit more exciting than waiting for magnesium or Alka-Seltzer to disappear.
Eventually, I came across a Flinn 1 experiment which focused on the reaction between sodium thiosulfate and hydrochloric acid.
Na 2 S 2 O 3 (aq) + HCl (aq) → 2NaCl (aq) + S (s) + H 2 O (l) + SO 2 (g)
What I liked most about this reaction was the easy and consistent timing mechanism it provided my students with, which could eliminate the ambiguity and differences in timing approaches that lab groups had used in the past.
Here’s how: As the reaction proceeds, one of the products is sulfur. As more sulfur gets produced, the solution becomes more and more cloudy until eventually the solution is opaque. Because of this, the moment that you can no longer see through the solution can be used as a consistent way to stop time. When I asked my students how we would all consistently decide on when the solution is opaque, many of them suggested to place some sort of object on the other side of the beaker so that we would all stop the timer when the object was no longer visible. This naturally progressed to the idea of drawing something on the beaker itself (an X on the bottom in this case) and applying the same reasoning.
reactions series of beakers with X on bottom

series of beakers after X is blocked

This reaction and the implementation of this natural clock can be seen below in a Flinn video 2 .
Even though it is just a matter of changing from visible to opaque, I noticed that the anticipation of waiting for that X to disappear had nearly all my students hovering over their beakers anxiously waiting to stop their timer. It even got to a point where different groups started to use their phones to make time lapse videos of their reaction beakers. You can see one below. As a teacher, it was fun to watch their level of excitement over something so seemingly simple.
Though I used this experiment to primarily investigate collision theory and different factors that affect the time it takes for a reaction to complete, it could easily be used to determine something more complex like reaction order ( see the entire Flinn video from which the above clip is taken ).
I also found this lab to serve as a great opportunity for my students to play a larger role in the creation of the experimental setup since there wasn’t much complexity to it. I facilitated the design of the experiment by asking my students a series of questions that were meant to feel like it was a genuine conversation happening between scientists interested in answering a question. The PowerPoint that I used to help facilitate this discussion can be found as Supporting Information at the bottom of this post if you are logged in to ChemEd X, but the general theme followed these questions:
- What is our independent variable? How should we go about changing this?
- Should the total volume of each beaker be the same or different? Why?
- What is our dependent variable?
- Are there any variables that we should control?
- How should we go about timing our reaction?
- How should we record and organize our data?
- How are we going to figure out our concentrations in terms of Molarity?
- What are we going to do with our data once we have it? Graph it?
I don’t include students in things like this often enough and it’s important that I continue to remind myself the beneficial experience this can provide for students to get a more accurate understanding of how science operates.
However you decide to do it, the general approach to this experiment goes something like this:
1) Using a Sharpie, draw a black X on the bottom (outside) of each beaker. 2) A stock solution of 0.15 M Na 2 S 2 O 3 is used to make 5 different concentrations using different amounts of distilled water, though our tap water worked just fine too. The total volume of each solution should be the same in each beaker. 3) Add 5 mL of 2 M HCl to your first beaker to start the reaction. You can give it an initial stir to uniformly distribute the HCl. The timer starts after this initial swirl. 4) While looking down at the beaker, stop the timer the moment you see the X completely disappear from sight. 5) Do this for all your samples and start analyzing your data
After everyone had finished the experiment and analyzed their results, I was thrilled to see that the data from each group produced a graph that displayed the relationship I was looking for. Not a single group had one weird outlier or a graph with seemingly random points all over the place! Some of the groups even paid close enough attention to the fact that each beaker had different levels of “opaqueness” to them. This provided a great opportunity to talk about the benefits of qualitative evidence as well. I attribute these consistent results to two primary things:
1) Consistent timing mechanism that each group can easily reproduce 2) It is almost impossible to mess up this reaction—you’re just pouring HCl into your Na 2 S 2 O 3 solution. Minimizing chances for experimental error was huge.
Effect of Concentration on Reaction Time Graph

Though I don’t always shoot for consistent data between groups when we do a lab, I knew that the arguments would vary between groups when trying to explain why their experiment displayed the relationship it did. It is the arguments I am most interested in developing after students complete their data analysis.

Students were tasked with developing their initial argument using a Claim, Evidence, Reasoning (CER) framework. Though most boards had similar claims, they often differed in what evidence they chose to present. They all had access to the same evidence and yet different groups intentionally left out certain pieces of evidence—why? Where their boards differed the most was in their reasoning, which is meant to have them justify why their evidence makes sense based on known scientific principles. I should mention that the students had not been presented anything about collision theory before this lab and yet many of them were able to come up with a valid particle-based explanation while others either circled around ambiguity, lacked detail, or simply displayed some form of misconception. The important part of this was that they tried their best, based on the models they had running around in their heads, to explain the phenomenon and knew that it was up to the scientific community (our class) to act as a filter for sorting out valid explanations from ones that either lacked detail or could not quite account for the evidence. This is the process I love doing the most.
The lab itself took about 30 mins to do but because I involved them in the experimental setup and dedicated time to construct arguments that were presented, debated, and refined, the entire process took 3 periods (1 hr each).
I want to thank Flinn for inspiring the idea for the experiment in the first place and NSTA’s book Argument-Driven Inquiry in Chemistry 3 for providing the framework we used to set up and make sense of the investigation.
Resources 1 Rate of Reaction of Sodium Thiosulfate and Hydrochloric Acid . N.p.: Flinn Scientific, n.d. Pdf . https://www.flinnsci.com/globalassets/flinn-scientific/all-free-pdfs/dc91860.pdf 2 "Rate of Reaction of Sodium Thiosulfate and Hydrochloric Acid..."20 Dec. 2012, & https://www.youtube.com/watch?v=r4IZDPpN-bk . Accessed 17 Jan. 2017. 3 "NSTA Science Store: Argument-Driven Inquiry in Chemistry: Lab ...." 1 Oct. 2014, https://www.nsta.org/store/product_detail.aspx?id=10.2505/9781938946226 . Accessed 17 Jan. 2017.
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016) .
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations .
Other Safety resources
RAMP : Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
Science Practice: Analyzing and Interpreting Data
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data.
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data. Analyze data using tools, technologies, and/or models (e.g., computational, mathematical) in order to make valid and reliable scientific claims or determine an optimal design solution.
Science Practice: Asking Questions and Defining Problems
Asking questions and defining problems in grades 9–12 builds from grades K–8 experiences and progresses to formulating, refining, and evaluating empirically testable questions and design problems using models and simulations.
questions that challenge the premise(s) of an argument, the interpretation of a data set, or the suitability of a design.
Scientific questions arise in a variety of ways. They can be driven by curiosity about the world (e.g., Why is the sky blue?). They can be inspired by a model’s or theory’s predictions or by attempts to extend or refine a model or theory (e.g., How does the particle model of matter explain the incompressibility of liquids?). Or they can result from the need to provide better solutions to a problem. For example, the question of why it is impossible to siphon water above a height of 32 feet led Evangelista Torricelli (17th-century inventor of the barometer) to his discoveries about the atmosphere and the identification of a vacuum.
Questions are also important in engineering. Engineers must be able to ask probing questions in order to define an engineering problem. For example, they may ask: What is the need or desire that underlies the problem? What are the criteria (specifications) for a successful solution? What are the constraints? Other questions arise when generating possible solutions: Will this solution meet the design criteria? Can two or more ideas be combined to produce a better solution?
Science Practice: Constructing Explanations and Designing Solutions
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories. Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future.
Science Practice: Developing and Using Models
Modeling in 9–12 builds on K–8 and progresses to using, synthesizing, and developing models to predict and show relationships among variables between systems and their components in the natural and designed worlds.
Modeling in 9–12 builds on K–8 and progresses to using, synthesizing, and developing models to predict and show relationships among variables between systems and their components in the natural and designed worlds. Use a model to predict the relationships between systems or between components of a system.
Science Practice: Engaging in Argument from Evidence
Science practice: obtaining, evaluating, and communicating information.
Engaging in argument from evidence in 9–12 builds on K–8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about natural and designed worlds. Arguments may also come from current scientific or historical episodes in science.
Engaging in argument from evidence in 9–12 builds on K–8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about natural and designed worlds. Arguments may also come from current scientific or historical episodes in science. Evaluate the claims, evidence, and reasoning behind currently accepted explanations or solutions to determine the merits of arguments.
Science Practice: Planning and Carrying out Investigations
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models.
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models. Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly.
HS-PS1-5 Rates of Reactions
Students who demonstrate understanding can apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org .
Assessment is limited to simple reactions in which there are only two reactants; evidence from temperature, concentration, and rate data; and qualitative relationships between rate and temperature.
Emphasis is on student reasoning that focuses on the number and energy of collisions between molecules.
All comments must abide by the ChemEd X Comment Policy , are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 11.

This is awesome. I found this lab to be very useful too, and appreciate how you've shared how it's run in your classroom.

Thanks! Glad I came across it and was able to reflect/share.
Bringing Kinetics to First Year Chem

I thoroughly enjoyed reading your reflections on this activity. I use a microscale version of this reaction in my AP Chemistry class and have students calculate the order of reaction with respect to thiosulfate and hydrochloric acid. It is a very reliable procedure and the students enjoy the lab for the reasons you've discussed.
I usually don't touch on kinetics in my first year course, but this year while I was teaching it I realized that the theory of kinetics (collision theory, activation energy, catalysts, decrease of rate with time) is very accessible to first year students who have a firm grasp of the particulate nature of matter. Thank you for posting how you went through this with them, I plan on giving it a shot in my chemical reactions unit that will now include basic kinetic theory.
Kinetics Question
Thank you for sharing this lab! I am a new teacher and really appreciate such good resources.
One question: In my textbook (Chemistry by Whitten, Davis, Peck, Stanley), the integrated rate laws use ln [A]0- ln [A]= a kt. So when working textbook problems, I've had the students use the coefficient in calculations. However, I noticed in the AP FRQ a is not included (such as 2004B #3) and a is not included in the given equations. I am confused on what is the correct method and how I should be teaching this. I would appreciate any clarification.
Integrated Rate Law Question
Hi Beverly,
I have a coppy of the 10th edition of Whitten (though I do not use it) and it does indeed use " a " for the coefficient from the balanced equation. This is new to me and I have not seen it before. However it makes sense if you look at how they set up the integration compared to other sources.
Method 1: The rate of reaction of a first order reaction A --> Products is defined as Rate = -d[A]/dt = k [A]. This assumes A has a coefficient of 1.
Method 2: The Whitten text defines the same thing, but uses the reaction a A --> Products as the model. This leads to Rate = (1/ a )(-d[A]/dt). This affects the value of k in Rate = k [A] and the inclusion of a in the integrated rate law.
k (the "rate constant") is simply a proportionality constant, it's value just depends on how you define it. If we say that k = ak' then if you're asked to calculate "the rate constant of the reaction" and use Method 1 exclusively then you are solving for k . If you take in to account the stoichiometry, you are solving for k' .
Given the prevelance of not including a I would assume that "the rate constant" is widely considered by chemists to be the value obtained via Method 1.
Now for your concerns about practice in AP Chemistry.
This area of possible confusion have only come up twice to my knowledge. Once in 2008 #3 and once in 2016 #5. In both situations the graders accepted either value for k . The scoring guidelines for both exams are here:
2008 Scoring Guidelines
2016 Scoring Guidelines
The forumula included on the formula chart, combined with the precedent of these two equations leads me to belive that either method will be accepted unless a more specific question were asked.
I hope this helps.
Definition of reaction rate

As defined by the International Union on Pure and Applied Chemistry, reaction rate depends on stoichiometry. You can find the defnition here: https://goldbook.iupac.org/html/R/R05156.html . So, if the reaction is aA --> products, the rate is defined as -(1/a)(d[A]/dt). This affects the integration, and therefore the integrated rate law, just as Kaleb says.. If the stoichiometry is A --> products, then a does not appear in the integrated rate law but only because a = 1. It appears that the AP folks allowed for both of these possibilities, which seems reasonable to me.
The version with a included is more general and gives the other version when a = 1. The distinction is important when rate constants are reported in a published paper because if the stoichiometric coefficient a is not included the rate constant value will be off by a factor of a. However, the distinction seems a lot less important when students are learning this for the first time.
I did want to clarify that the version with a included is the version that most chemists who do kinetics studies would say is correct.
IUPAC To the Rescue!
Thank you for your response and link to the Gold Book! I am glad to know that the chemistry community does have a a published, accepted standard for this (and that I was incorrect in my assertion). I agree that the distinction seems less imporant for first-time students, I am curious if this is the reasoning of the AP Test Development Committee as well and am going to reach out to see.
Kinetics Response
Hey Beverly,
I don't teach AP so I don't want to suggest a "correct method" but here's what I'm thinking based on my own limited knowledge of integrated rate laws.
The short answer: I don't think the coefficient ( a ) is necessary.
Why I think a isn't necessary: I think your answer can be found in the difference between differential rate laws and integrated rate laws--at least it helped me understand it better. Resource here
Differential rate laws express the rate of reaction as a function of a change in the concentration of one or more reactants over a particular period of time, they are used to describe what is happening at the molecular level during a reaction (mechanism-focused).
On the other hand, integrated rate laws express the reaction rate as a function of the intial concentration and a measured (actual) concentration of one or more reactants after a sepcific amount of time has passed--they are used to determine the rate constant and the reaction order from experimental data.
To me, that means that because the order of a reaction is determined experimentally, they do not represent the coefficients from a balanced equation like they would for an equilibrium expression. In other words, the expression used for a rate law generally bears no relation to the reaction equation, and must be determined experimentally (Resource here )
I hope that helped somewhat. There are several people on this site that would be most likely provide a much easier answer so I can reach out to others if this didn't help. If nothing else, I got to brush up on topics I haven't dealt with for some time!
sodium thiosulfate pentahydrate
I checked my chemical inventory and found that I only have the hydrate. Do you think it would work?
Hydrate Will Work
It will work. Just make sure you account for the added mass from water when making your solutions of desired concentration.
Disappearing X

Great minds think alike. I posted a video post about 1.5 weeks before on this same topic.
https://www.chemedx.org/blog/disappearing-x-lab
I plan on reading your post more in depth tonight during conferences if time allows. I don't do much modeling or CER although more of this may show up as we revamp our chemistry 1 curriculum to comply with our updated state science standards.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
Practical videos | 14–16 years
- 1 Access free videos to support your teaching
- 2 Paper chromatography
- 3 Rates of reaction
- 4 Simple distillation
- 5 Enthalpy change of combustion
- 6 Conservation of mass
- 7 Electrolysis of aqueous solutions
- 8 Halogen displacement reactions
- 9 Identifying ions
- 10 Preparing a soluble salt
- 11 Reactivity series of metals
- 12 Simple titration
- 13 Temperature change (neutralisation)
- 14 Potable water
- Rates of reaction

- No comments
Observe how the concentration of sodium thiosulfate solution affects its rate of reaction with hydrochloric acid
The value of experiencing live practical work cannot be overstated. Numerous studies provide evidence of its value in terms of learner engagement, understanding, results and the likelihood of continuing to study chemistry or work in a related field.
Use this video to complement live practical work, or to help learners understand the methods, equipment and skills when they cannot access the lab.
Source: © Royal Society of Chemistry
Investigate rates of reaction (observing a colour change) using this video, including a step-by-step method, calculation support for learners and evaluation
Chapter titles: 00:09 Introduction to rates of reaction; 01:34 Carrying out the experiment; 05:45 Calculations; 07:40 Evaluating the method.
- Teacher notes
Full teacher notes are available in the supporting resources booklet (also available in MS Word ), including ideas for how to use this video and the accompanying activites and answers to use as part of your teaching.
Get the resources

Supporting resources
Detailed teacher notes, learner activities and answers

Learner slides
Integrated instructions, Frayer model and Johnstone's triangle

- Technician notes
Equipment, chemicals, hazards and disposal information
Notes on running the practical experiments
Ask learners to work in pairs. Demonstrate how to set up the equipment first (either in-person or via the video) to emphasise health and safety issues. Remind learners that they must wear eye protection and direct them to the relevant student safety sheets (SSS).
It is important that all learners have access to a stop bath to dispose of their waste products as sodium thiosulfate solution ( SSS034 ) reacts with hydrochloric acid ( SSS020 ) to toxic produce sulfur dioxide gas. Explain that the stop bath is a solution of sodium carbonate/hydrogen carbonate ( SSS033 ) with an acid–base indicator such as phenolphthalein ( SSS070 ). It neutralises any remaining acid and the sulfur dioxide reacts with the water to produce sulfuric acid. If the indicator is showing the acidic colour, refresh the stop bath by adding more sodium carbonate solution. Ensure the room is well ventilated. A microscale version of the experiment is available from CLEAPSS.
Teaching rates of reaction at 16–18 too? Watch the practical video to show learners how to monitor the rate of reaction and identify the effects of changing temperature and concentration, using both initial rate and continuous monitoring methods. Plus, download the resources for teacher and technician notes, follow-up worksheets and more.

Health, safety and technical notes
Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Refer to SSERC/CLEAPSS Hazcards, recipe books and student safety sheets. Hazard classification may vary depending on supplier. Download the technician notes for the full equipment list, safety notes and disposal information.
Measure 10 cm 3 of sodium thiosulfate and pour it into the conical flask.
Measure 40 cm 3 of distilled water and add it to the conical flask.
Place the flask on the black cross.
Using a clean measuring cylinder, measure 10 cm 3 of hydrochloric acid.
Add the acid to the flask, start the stop clock and swirl.
Place the watch glass on top of the flask to limit breathing in sulfur dioxide gas.
Time how long it takes until you can no longer see the black cross. Look at the cross from a distance of at least 20 cm above the top of the flask.
Repeat the method using 20, 30, 40 and 50 cm 3 of sodium thiosulfate solution with 30, 20, 10 and 0 cm 3 of distilled water.
Find the integrated instructions for this experiment in the PowerPoint slides .
Real-world contexts
- Highlight the importance of understanding rates of reaction to avoid dust explosions in food production, such as flour mills. Demonstrate how surface area affects rate with the Exhibition chemistry video Powder power .
- Link to careers, such as Misbah’s role as a senior principal scientist . She is a computational chemist and predicts which catalysts will be best at removing harmful pollutants from vehicles.
- Read this CPD article for rates of reaction teaching tips and contexts, such as fighting infections, alcohol metabolism and more.
Learners will need to have a clear understanding of the following scientific terminology:
- Rate of reaction – the change in the concentration of the reactant or product per unit time.
- Chemical change – a change in which one or more new substances are formed.
- Reactant – a starting substance in a chemical reaction.
- Product – a substance made during a chemical reaction.
- Mass – a measure of the amount of matter in a substance.
- Precipitate – an insoluble solid that forms when two solutions react.
- Collision theory – for a chemical reaction to occur, two or more particles must collide in the correct orientation (the right way around) and with enough energy to break the bonds.
- Activation energy – the minimum amount of energy required for a reaction to occur.
- Temperature – a measure of how hot or cold an object is.
- Concentration – the mass of solute dissolved in a fixed volume of solvent.
- Pressure – the force per unit area.
- Catalyst – a substance that changes the rate of reaction without ‘taking part’ in the reaction.
- Surface area – the area of a solid chemical substance used in a chemical reaction.
- Volume – a measure of the amount of liquid.
You will find a template, example Frayer model and suggested answers for the term ‘collision theory’ in the PowerPoint slides . Find more examples and tips on how to use Frayer models in your teaching.
More resources
- Investigate the volume of hydrogen gas produced when magnesium reacts with hydrochloric acid .
- Use the Sustainability in chemistry resource on Catalysts and reaction conditions for sustainable industry to give your learners an authentic insight into how scientists work. Plus, download the research and presentation activity.
- Practise Interpreting rates of reaction graphs with your 14–16 year-old learners with this lesson plan and student worksheets.
Common misconceptions
Difficulties related to chemical change.
- Misconception: if you can’t see it, it has disappeared or was never there. Avoid using language surrounding chemical change that confuses learners, eg in this practical try to avoid the phrase ‘the cross has disappeared’ instead use the phrase ‘you can no longer see the cross’.
- Misconception: all reactions are driven by two reactants. Demonstrate reactions with more and less, eg decomposition, than two reactants.
- Misconception: all reactants equally influence how fast a reaction proceeds. Introduce limiting reactants.
Application of collision theory
Learners can struggle to form a full answer including:
- How the changing variable affects the reactants’ behaviours/properties.
- How this relates to collision theory.
- The concept of rate (per unit of time).
There is a lot going on here, so it is important to provide a clear scaffold for learners. Initially, the use of a structure strip (see the example and suggested answer in the supporting resources ) will help, followed by plenty of practice providing learners with similar but slightly different questions.
There are several misconceptions around the understanding of catalysts including:
- Catalysts ‘are not involved’ in chemical reactions.
- Catalysts break chemical bonds.
- Catalysts increase the yield of the product.
- Catalysts only affect the rate of the forward reaction.
Be careful with your language. The definition in some pre-16 specifications eg ‘catalysts change the rate of reaction but are not used up during the reaction’ can lead to misconceptions if the definition is not carefully unpacked and linked to the reaction profile, showing an alternative reaction pathway with a lower activating energy. This 5-minute demonstration clearly shows the catalyst is involved in the reactions as the colour changes from pink to green and back to pink.
Cross-curriculum links and skills
This practical activity provides the opportunity to develop several ‘working scientifically’ skills and mathematical skills including:
- Following instructions to carry out experimental techniques or procedures.
- Identifying and controlling significant quantitative variables where applicable, and planning approaches to take account of variables that you cannot readily control.
- Accurately measuring quantities and recording observations.
- Drawing a graph with a line/curve of best fit.
- Calculating the gradient of a graph by drawing a tangent to the curve.
- Using the correct units and levels of accuracy when carrying out chemical calculations.
If asked to draw another line on a graph (eg, volume of gas produced (y-axis) versus time (x-axis)) learners can struggle to know how to draw it. Should the start go up more steeply? Where does it plateau? Encourage learners to tell the story of their graph. Linking what is happening in the reaction vessel and their observations to what the graph is showing. Using a Johnstone’s triangle approach can also help here. Download the PowerPoint slides for an example and suggested answers.
Drawing a tangent to a graph
Many learners find this challenging and depending on when you teach rates, they may be meeting the concept for the first time. Talk to your maths colleagues to find out when they teach tangents.
Rates of reaction supporting resources
Rates of reaction technician notes, rates of reaction slides, additional information.
The original video script, supporting resources and slides were written by Dorothy Warren. The technician notes were adapted by Sandrine Bouchelkia.

More Dorothy Warren

Simple distillation | practical videos | 14–16 years
Teaching the bonding spectrum

Boost your teaching practice with subject-specific personal development

More Sandrine Bouchelkia

Enthalpy change of combustion of ethanol | practical videos | 14–16 years

Paper chromatography | practical videos | 14–16 years

Access free videos to support your teaching

Paper chromatography

Simple distillation

Enthalpy change of combustion

Conservation of mass

Electrolysis of aqueous solutions

Halogen displacement reactions

Identifying ions

Preparing a soluble salt

Reactivity series of metals

Simple titration

Temperature change (neutralisation)

Potable water
- 14-16 years
- Practical experiments
- Practical skills and safety
- Observing and measuring
- Recording data
- Interpreting data
Related articles

Rates of reaction | practical videos | 16–18 students
By Dorothy Warren , David Paterson and Sandrine Bouchelkia Five out of five
Video and supporting resources, includes an initial rate (iodine clock reaction) and continuous monitoring method (volume of gas)

Chromatography challenge | 16–18 years
By Andy Markwick
Explore analytical techniques and their applications with a chromatography investigation and research activity

Demonstrate concentration and density with a transition metal colloid cell
2024-02-19T10:06:00Z By Declan Fleming
Boost 11–14 learners’ understanding of diffusion and transition metal chemistry
No comments yet
Only registered users can comment on this article., more practical.
By Karen Marshall and Sandrine Bouchelkia
Video and resources investigating the heat energy change of combustion of ethanol
By Dorothy Warren and Sandrine Bouchelkia
Video and resources showing how to separate water from a coloured solution
Video and resources showing how to separate colours in inks using paper chromatography
- Contributors
- Email alerts
Site powered by Webvision Cloud
Rates - Thiosulfate ( OCR A Level Chemistry )
Revision note.

PAG 10.2: Rates – Thiosulfate & Acid
The disappearing cross experiment.
- A simple experiment which can be done to determine how the rate of reaction is affected by concentration is the disappearing cross experiment
- This experiment can be done for a number of different reactions, but the following reaction is commonly used:
Na 2 S 2 O 3 (aq) + 2HCl (aq) → 2NaCl (aq) + H 2 O (l) + SO 2 (g) + S (s)
- In this reaction, sodium thiosulphate reacts with hydrochloric acid
- The key product in this experiment is the solid sulfur which causes the solution to become opaque
- Changing the concentration - this can be for the hydrochloric acid or the sodium thiosulfate solution
- Changing the temperature (OCR tend to use this one to calculate the activation energy of a reaction)
- The disappearing cross experiment can be used for any reaction where a solid is produced as one of the products, as this will cause the solution to become cloudy.
- It does not have to be done in a conical flask, it could be done in a test tube with the cross placed underneath / behind the test tube.
- Careful: This is a very simple experiment, but exam questions can take it in many different directions from a relatively straight forward rate calculation to the more complex Arrhenius plot and equation to determine activation energy
- Measure 50.0 cm 3 of sodium thiosulfate solution into a conical flask
- Place the conical flask on the black cross
- Measure 5.0 cm 3 of dilute hydrochloric into a measuring cylinder
- Add the hydrochloric acid to the conical flask and immediately start the stopwatch
- Swirl the mixture
- Stop the stopwatch when the cross disappears and record the time
- Repeat the experiment for a minimum of four more / different concentrations
Dilution of sodium thiosulfate solution table
| ) | ) | |
| 10 | 40 | 0.2 |
| 20 | 30 | 0.4 |
| 30 | 20 | 0.6 |
| 40 | 10 | 0.8 |
| 50 | 0 | 1.0 |
- Careful: The process is the same as outlined in the table above where the total volume must remain constant when diluting

Diagram showing the apparatus needed to investigate reaction rate in the disappearing cross experiment
Specimen Results
- Results could be expressed in terms of relative dilution of the sodium thiosulfate solution (less common) or in terms of concentration of the sodium thiosulfate solution
| 0.2 | 115.2 |
| 0.4 | 57.6 |
| 0.6 | 30.0 |
| 0.8 | 15.6 |
| 1.0 | 7.2 |
| ) | |
| 0.05 | 115.2 |
| 0.10 | 57.6 |
| 0.15 | 30.0 |
| 0.20 | 15.6 |
| 0.25 | 7.2 |
- These results could be used in a number of different ways:
- Plot a graph of the results with the concentration of sodium thiosulfate on the x-axis and time on the y-axis
- Increasing the concentration of the sodium thiosulfate solution increases the rate of reaction
- This means that the time for the cross to disappear decreases
- The shape of the plotted graph will indicate if the reaction is 0, 1 or 2 order
- If this reaction was performed at different temperatures instead of with different concentrations, then the results could be manipulated, plotted and used to calculate the activation energy for this reaction
You've read 0 of your 0 free revision notes
Get unlimited access.
to absolutely everything:
- Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Join the 100,000 + Students that ❤️ Save My Exams
the (exam) results speak for themselves:
Did this page help you?
Author: Richard
Richard has taught Chemistry for over 15 years as well as working as a science tutor, examiner, content creator and author. He wasn’t the greatest at exams and only discovered how to revise in his final year at university. That knowledge made him want to help students learn how to revise, challenge them to think about what they actually know and hopefully succeed; so here he is, happily, at SME.

Concentration affecting Reaction Rate
- Show Solutions 1. Sulfur precipitate forms. 2. The time taken for the precipitate to form will indicate the speed of the reaction. 3. faster 4. Increasing the concentration of sodium thiosulfate will reduce the reaction time. 5. As the concentration of sodium thiosulfate increases, the particles become closer and there is an increase chance of them colliding with the hydrochloric acid particles and reacting. Therefore the effect of increasing the concentration is to increase the rate of reaction

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.

- Pure Chemicals
- Standard Solutions
- Deionised Water
- Distilled Water
- Ultrapure Water
- Absolute Ethanol
- Demineralised Water
- Propan-2-ol/Isopropanol
- Why Buy From ReAgent?
- Good Manufacturing Practice
- Investors In People (IIP)
- ISO 9001:2015 Accreditation
- ISO 13485:2016 Accreditation
- ISO 14001:2015 Accreditation
- ISO 45001: 2018 Accreditation
- Living Wage & Living Hours
- Our Memberships
- Our Customers
- Environment Policy
- Quality Policy
- Smoke Free Policy
- Production Facilities
- Bespoke Chemical Services
What Happens When Hydrochloric Acid and Sodium Thiosulphate React?
- The Chemistry Blog
- Posted on June 21, 2018
Jessica Clifton
When hydrochloric acid (HCl) and sodium thiosulphate (Na 2 S 2 O 3 ) are combined, an interesting reaction takes place and the colourless solution turns opaque. But why does this happen, and how can we use this opacity to determine the rate of reaction?
In this post:
The Reactants
The chemicals used in this experiment are both extremely important in their own areas. In case you missed our previous posts, hydrochloric acid is a strong acid that plays an important role in a range of industries. From regenerating cation exchange resins to neutralising the pH of swimming pools, it is a workhorse chemical that is used in nearly every industry.
Sodium thiosulphate is a chemical that has been classified by the World Health Organisation as one of the most effective and safe medicines needed in the health system. An efflorescent compound that appears as a colourless pentahydrate, sodium thiosulphate is used as a medication for things like cyanide poisoning and pityriasis versicolour.
While these compounds have crucial impacts in their separate applications, when they come together they provide a perfect example of how the rate of a reaction increases, decreases and how it can be measured.
What is the Rate of a Reaction?
A reaction happens when particles collide, resulting in the reactants getting consumed and new products getting formed. Therefore, in order for a reaction to be successful, the collisions have to have sufficient energy. The greater the number of particles, the more energy these collisions will create. This means that the concentration of the reactants directly affects the energy of a reaction.
With this in mind, the rate of a reaction can be defined as an increase or decrease of concentration in any one of the reactants or final product.
As the concentration of a reactant increases, for example, the number of reacting molecules increases. This means that there is a greater number of collisions which leads to a quicker reaction time and a larger rate of reaction.
Therefore, although there is an inverse relationship between concentration and the rate of a reaction, it is a relationship that is directly proportional. This concept is best demonstrated by the reaction between hydrochloric acid and sodium thiosulphate.

The Reaction
When sodium thiosulphate is added to a solution of hydrochloric acid, an insoluble precipitate of sulphur (S) is formed. Sulphur dioxide (SO 2 ) and water (H 2 O) are also formed, but it is the solid sulphur that has the biggest impact here.
The sulphur is a colloid in this reaction, staying in suspension and eventually blocking the light from reaching the solution. This transforms the solution from being colourless to being milky and entirely opaque. This happens because of the precipitates of elemental sulphur that are being formed, which are insoluble and eventually cloud the water. You can see this by drawing an X on a piece of paper, placing it under your beaker and watching as it begins to disappear.
If the concentration of sodium thiosulphate is high, the solution will cloud fairly quickly (generally between 15-30 seconds). If the concentration of sodium thiosulphate is low, then it will take longer for the reaction to occur. This is how you can measure the rate of the reaction.
Measuring the Rate of Reaction
The rate of the reaction can be studied by measuring the opaqueness of the solution against the time taken for it to change. Changing the concentration of sodium thiosulphate will change the time it takes for a certain amount of sulphur to form and, therefore, how long it takes for the solution to turn cloudy.
You can lower the concentration of sodium thiosulphate by diluting it with distilled water. This will reduce the number of Na s S 2 O 3 particles which ultimately means fewer collisions. The sulphur precipitates will then appear at a lower rate. This means a longer reaction time and smaller reaction rate.
Comparatively, a reaction that uses a very low concentration of sodium thiosulphate may take up to 5 minutes for the solution to become fully opaque.

ReAgent’s online chemical shop has top-grade hydrochloric acid and sodium thiosulphate products available. We’re internationally recognised for our first-class customer service, industry leading quality and commitment to the environment. When you buy with us, you’re buying chemicals that are backed by a 100% quality guarantee. Order online today or speak to our friendly team about any queries you may have.
About the author
The blog on chemicals.co.uk and everything published on it is provided as an information resource only. The blog, its authors and affiliates accept no responsibility for any accident, injury or damage caused in part or directly from following the information provided on this website. We do not recommend using any chemical without first consulting the Material Safety Data Sheet which can be obtained from the manufacturer and following the safety advice and precautions on the product label. If you are in any doubt about health and safety issues please consult the Health & Safety Executive ( HSE ).
How can ReAgent help you?
- By using this form you agree with the storage and handling of your data by this website.

- Top Navigation

Looking to buy Hydrochloric Acid?
- Information
- Specification
- Hazards & Safety

- Study Abroad Get upto 50% discount on Visa Fees
- Top Universities & Colleges
- Abroad Exams
- Top Courses
- Read College Reviews
- Admission Alerts 2024
- Education Loan
- Institute (Counselling, Coaching and More)
- Ask a Question
- College Predictor
- Test Series
- Practice Questions
- Course Finder
- Scholarship
- All Courses
- B.Sc (Nursing)
Rate of Reaction Between Sodium Thiosulphate & Hydrochloric Acid

Jasmine Grover
Senior Content Specialist
Rate of reaction plays a significant role in the chemical reaction because it tells us the speed of the reaction. Rate of reaction is referred to as the speed with which reactants are converted into products at a given time. In other words, rate of reaction is defined as the change in concentration of reactants or products per unit time. There are many factors which affect the rate of reaction such as the nature of the reactant, presence of catalyst, presence of radiation, surface area of the reactants, temperature etc. One such factor is the concentration which is directly proportional to the rate of reaction.
Read More : Concepts in Chemistry
|
|
Key Terms: Rate of Reaction, Reactants, Products, Catalyst, Concentration, Sodium Thiosulphate, Hydrochloric Acid
[Click Here for Sample Questions]
To study the effect of concentration on the rate of reaction between sodium thiosulphate and hydrochloric acid.
Apparatus Required
The materials/ apparatus used in the given experiment are as follows:
- Five conical flasks - 100 ml
- One measuring cylinder - 50 ml
- One pipette - 10 ml
- One burette
- One plain paper
- One white tile
- One stopwatch
- Chemicals: Sodium thiosulphate (0.2M), Hydrochloric acid (1M)
|
|
|
|
The effect of concentration on the reaction rate is governed by the law of mass action . As per this law, the rate of reaction is directly proportional to the product of molar concentration of the reactants. Moreover, with the increase or decrease in the concentration of the reactants the rate of reaction increases or decreases. When sodium thiosulphate reacts with hydrochloric acid then it forms the elemental sulphur accompanied by the evolution of sulphur dioxide gas. The reaction that takes place is written as follows:
Na 2 S 2 O 3 (aq) + 2HCL (aq) \(\rightarrow\) 2NaCl (aq) + SO 2 (g) + H 2 O (l) + S (s)
Furthermore, the rate of disappearance or consumption of sodium thiosulphate (Na 2 S 2 O 3 ) or the rate of precipitation of elemental sulphur tells us the rate of reaction. It is more convenient to determine the reaction by the rate of precipitation of sulphur which imparts the turbidity to the reaction mixture. Also, the rate of reaction can be studied by measuring the time taken to form enough sulphur to make some mark invisible on the paper, kept under the conical flask in which the reaction is carried out. Therefore, with the increase in the concentration of the reacting species, the rate of precipitation of sulphur also rises. As a result, the molecular collisions per unit time of reactants increase which increases the chances of product formation. If the product formation increases then the rate of reaction will also increase.
To determine the effect of concentration on rate of reaction follow the given steps:
- Initially, take the five flasks (each 100ml). Wash them properly with water and mark them as 1,2,3,4,5.
- Now, put the 50ml, 40ml, 30ml, 20ml and 10ml of 0.2M sodium thiosulphate (hypo) solution in the flasks marked 1,2,3,4 and 5 respectively.
- Then, put 10ml, 20ml, 30ml and 40ml of distilled water in conical flasks 2,3,4 and 5. Now, each flask contains the 50 ml solution i.e. hypo solution + distilled water.
- Now, take a pipette out 10 ml of 1M HCl solution in the flask no.1 and then place it on a white sheet of paper having a mark of ‘X’ as shown in figure below.
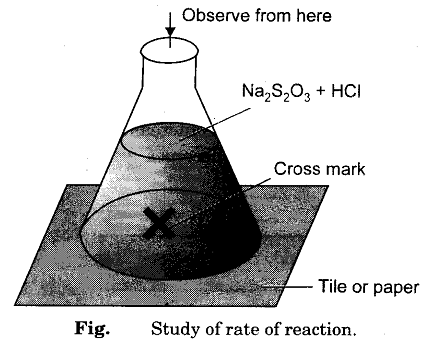
- After that, immediately start the stopwatch.
- Observe the contents of the flask from the top and note down the time taken for the disappearance of the cross marked on the paper due to formation of the elemental sulphur in the flask which makes the solution opaque.
- Repeat this experiment by taking conical flasks no.2 to no.5 in the order as well as adding 10 ml of 1M HCl solution in each flask.
Observation Table
The readings of the given experiment is recorded in the tabular form so that calculations can be done easily.
Volume of 1M HCl solution added to each conical flask = 10 ml
| 50 ml | - | t1 |
| 40 ml | 10 ml | t2 |
| 30 ml | 20 ml | t3 |
| 20 ml | 30 ml | t4 |
| 10 ml | 40 ml | t5 |
From the observation table, it is concluded that the time taken for disappearance of the mark ‘X’ should increase with the decrease in the concentration of sodium thiosulphate solution ( from 50 ml to 10 ml in flask no.1 to no.5 in the same order). The trend is shown below:
t 5 > t 4 > t 3 > t 2 > t 1
The graph between the concentration of the sodium thiosulphate and time taken for the disappearance of the mark 'X’ is plotted as:
Result
From the observation table and trend observed in the time taken for the disappearance of the mark ’X’ , it is clear that the rate of reaction between sodium thiosulphate and hydrochloric acid decreases with the decrease in the concentration of the sodium thiosulphate.
Precautions
There are some basic precautions that should be followed while performing the experiment:
- The volume of the distilled water, sodium thiosulphate and HCl should be measured accurately.
- The cross marked on the paper should be observed from the same height.
- The time taken for the disappearance of the mark ‘X’ should be noted carefully.
- Wash the apparatus properly.
|
|
|
|
|
|
Things to Remember
- Rate of reaction tells us the speed of the chemical reaction.
- The rate of reaction is directly proportional to the concentration of the reactants as per the definition of the law of mass action.
- With the increase in the concentration, the rate of reaction rises.
- With the decrease in the concentration, the rate of reaction also decreases.
- Rate of reaction can be determined by finding the rate of precipitation of sulphur.
Sample Questions
Ques. What are the units of the rate of a reaction? (2 marks)
Ans. There are two main units of expressing the rate of reaction. These are:
- Moles per litre per second \(\rightarrow\) Mol L -1 s -1
- Moles per litre per minute \(\rightarrow\) Mol L -1 min -1
However, Mol L -1 s -1 is widely used in chemistry.
Ques. Is it possible to determine the rate of reaction for all the reactions? (1 mark)
Ans. No, it is not possible for all the reactions. The rate of reaction for very slow and fast reactions cannot be determined.
Ques. What do you mean by hypo solution? (1 mark)
Ans . An aqueous solution of sodium thiosulphate (Na 2 S 2 O 3 .5H 2 O) is known as hypo solution.
Ques. What is the order of reaction involving reaction between sodium thiosulphate and hydrochloric acid? (3 marks)
Ans. The first order reaction is involved between the sodium thiosulphate and hydrochloric acid. It is because of the lower concentrations of the chemicals.
Na 2 S 2 O 3 (aq) + 2HCl (aq) \(\rightarrow\) 2NaCl (aq) + SO 2 (g) + H 2 O (l) + S (s)
Here, the rate of a reaction is first order with respect to sodium thiosulphate (Na 2 S 2 O 3 (aq)) as well as hydrochloric acid(HCl).
Ques. What is the nature of the rate of a reaction with time? (2 marks)
Ans. The rate of a reaction decreases with the passage of time because concentrations of the reactants are high in the beginning. As the time increases, the concentration of the reactants becomes low which slows down the reaction. As a result, the rate of a reaction goes down with time.
Ques. Why should air-free water be used for preparation of sodium sulphite solution? (2 marks)
Ans. Air free water should be used for preparation of sodium sulphite solution because sodium sulphite is easily oxidised to sulphate by dissolving air in the water. Therefore, air free water should be used to prepare aqueous solution of sodium sulphite.
Ques. Write down the principle involved in the study of the rate of a reaction between sodium thiosulphate and hydrochloric acid? (2 marks)
Ans. In the reaction between sodium thiosulphate and hydrochloric acid, the elemental sulphur i.e. colloidal sulphur is produced which results in the appearance of the turbidity in the solution. Also, it appears as when the cross marked on the paper disappears.
Ques. What do you understand from the rate of a reaction? (3 marks)
Ans. The rate of speed at which the reactants convert into products at a given time is known as the rate of a reaction. It can be represented as;
A \(\rightarrow\) B
where, A refers to the reactant and B refers to the product
Rate of a reaction = Decrease in concentration of A / Time taken
Rate of a reaction = - \(\Delta \) [A] / \(\Delta\) t
Rate of a reaction = Increase in concentration of B / Time taken
Rate of a reaction = + \(\Delta \) B / \(\Delta\) t
|
|
|
|
|
|
|
|
|
|
|
|
CBSE CLASS XII Related Questions
1. write the nernst equation and emf of the following cells at 298 k : (i) mg(s) | mg 2+ (0.001m) || cu 2+ (0.0001 m) | cu(s) (ii) fe(s) | fe 2+ (0.001m) || h + (1m)|h 2 (g)(1bar) | pt(s) (iii) sn(s) | sn 2+ (0.050 m) || h + (0.020 m) | h 2 (g) (1 bar) | pt(s) (iv) pt(s) | br 2 (l) | br - (0.010 m) || h + (0.030 m) | h 2 (g) (1 bar) | pt(s)., 2. discuss briefly giving an example in each case the role of coordination compounds in: biological systems medicinal chemistry analytical chemistry extraction/ metallurgy of metals, 3. how would you account for the following: of the d 4 species, cr 2+ is strongly reducing while manganese(iii) is strongly oxidising. cobalt(ii) is stable in aqueous solution but in the presence of complexing reagents it is easily oxidised. the d 1 configuration is very unstable in ions., 4. name the oxometal anions of the first series of the transition metals in which the metal exhibits the oxidation state equal to its group number., 5. write the mechanism of the following reaction : nbubr+kcn→etoh-h 2 o nbucn , 6. accomplish the following conversions: (i) nitrobenzene to benzoic acid (ii) benzene to m-bromophenol (iii) benzoic acid to aniline (iv) aniline to 2,4,6-tribromofluorobenzene (v) benzyl chloride to 2-phenylethanamine (vi) chlorobenzene to p-chloroaniline (vii) aniline to p-bromoaniline (viii) benzamide to toluene (ix) aniline to benzyl alcohol., subscribe to our news letter.


COMMENTS
2. action of Sodium Thiosulfate and Hydrochloric Acid continuedDiscussionSodium thiosulfate react. ion 1).Na2S2O3(aq) + 2HCl(aq) → S(s) + SO2(g) + 2NaCl(aq) Equation 1The kinetics of the reaction can be analyzed by graphing the. oncentration of Na2S2O3 as a function of both reaction time and 1/time. A plot of concentration versus time gives a ...
Procedure. Put 50 cm 3 of sodium thiosulfate solution in a flask. Measure 5 cm 3 of dilute hydrochloric acid in a small measuring cylinder. Add the acid to the flask and immediately start the clock. Swirl the flask to mix the solutions and place it on a piece of paper marked with a cross. Look down at the cross from above.
The aim of this experiment - Understanding the effect of concentration on the rate of reaction between hydrochloric acid and sodium thiosulphate. Theory: The reaction between Sodium thiosulphate (Na 2 S 2 O 3) and hydrochloric acid (HCl) To produce a colloidal solution of sulphur, where the solution obtained is translucent. The reaction ...
Put 10 cm 3 of sodium thiosulfate solution and 40 cm 3 of water into a conical flask. Measure 5 cm 3 of dilute hydrochloric acid in a small measuring cylinder. Warm the thiosulfate solution in the flask if necessary to bring it to the required temperature. The object is to repeat the experiment five times with temperatures in the range 15-55 °C.
A close up view of the classic experiment between Hydrochloric Acid and Sodium Thiosulfate (producing a sulfur precipitate), with varying concentrations of s...
sodium thiosulfate + hydrochloric acid → sodium chloride + water + sulfur dioxide + sulfur. Na 2 S 2 O 3 (s) + 2HCl ... add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask.
In this video, a series of experiments are used to show the effect of concentration on the rate of reaction between sodium thiosulfate and hydrochloric acid....
Na2S2O3 (aq) + 2HCL (aq) → H2O (l) + SO2 (g)+ 2 NaCl (aq) + S (s) It was observed that in a reaction, with an increase in the concentration of sodium thiosulphate gradually while keeping the concentration of hydrochloric acid constant, the rate of reaction has increased slowly. If "t" is the amount of time taken for the products to form ...
A more detailed experiment. A commonly used experiment to show the effect of concentration on rate is between dilute hydrochloric acid and sodium thiosulfate solution. Na 2 S 2 O 3 (aq) + 2HCl (aq) 2NaCl (aq) + SO 2 (g) + S (s) + H 2 O (l) At this stage, the only place you are likely to come across sodium thiosulfate is in this reaction.
Procedure. Put 50 cm3 of sodium thiosulfate solution in a flask. Measure 5 cm3 of dilute hydrochloric acid in a small measuring cylinder. Add the acid to the flask and immediately start the clock. Swirl the flask to mix the solutions and place it on a piece of paper marked with a cross. Look down at the cross from above.
Vary the concentrations of reactants and measure the time it takes for product to appear.This video is part of the Flinn Scientific Best Practices for Teachi...
avoid contact with the skin. gases escaping from reaction. may damage skin and eyes. place cotton wool at opening of conical flask to minimse gas escape. hot sodium thiosulfate solution. burns to the skin. do not heat above 60°C. sulfur dioxide. irritation to the eyes and lungs, particularly to people with asthma.
The aim for this investigation is to investigate what is the effect of the concentration of sodium thiosulphate (Na2S2O3) on the rate of reaction with Hydrochloric Acid (HCl). In my hypothesis statement, I stated "when the concentration of sodium thiosulphate increasing, the rate of reaction will increase, the less time taken for the „O ...
Transfer the hot sodium thiosulfate into the conical flask. Record the temperature of the hot sodium thiosulfate solution. Measure out 5cm 3 of the hydrochloric acid into the 10 cm 3 measuring cylinder. Place the conical flask onto the cross. Add the hydrochloric acid to the conical flask. Swirl the conical flask to mix the contents, at the ...
The Disappearing 'X' Experiment investigates how a change in concentration of sodium thiosulfate affects the rate at which it reacts with hydrochloric acid. By measuring the time it takes for the ...
The purpose of this demonstration is to investigate the effect of sodium thiosulfate concentration on the rate of reaction of sodium thiosulfate with hydrochloric acid. The reaction, which produces solid sulfur, will be followed by measuring the time needed for the reaction mixture to become opaque. The results will be analyzed graphically to ...
Whether you are introducing collision theory or something more demanding like reaction order, the reaction between sodium thiosulfate—Na 2 S 2 O 3 and hydrochloric acid can provide a consistent, accurate, and engaging opportunity for investigating these topics.. A few weeks ago, I was looking for a new reaction that could be used to investigate how concentration affects reaction time.
In this video, a series of experiments are used to show the effect of temperature on the rate of reaction between sodium thiosulfate and hydrochloric acid.On...
Measure 10 cm 3 of sodium thiosulfate and pour it into the conical flask. Measure 40 cm 3 of distilled water and add it to the conical flask. Place the flask on the black cross. Using a clean measuring cylinder, measure 10 cm 3 of hydrochloric acid. Add the acid to the flask, start the stop clock and swirl.
This experiment can be done for a number of different reactions, but the following reaction is commonly used: Na2S2O3 (aq) + 2HCl (aq) → 2NaCl (aq) + H2O (l) + SO2 (g) + S (s) In this reaction, sodium thiosulphate reacts with hydrochloric acid. The key product in this experiment is the solid sulfur which causes the solution to become opaque.
4. Add 5 cm 3 of the hydrochloric acid and at the same time start the stopclock. 5. Viewing from directly above the flask, note the time taken for the cross marked on the paper to 'disappear' i.e. be obscured by the sulfur precipitating. 6. Repeat the experiment using different concentrations of sodium thiosulfate. 7. Record your results in a ...
When hydrochloric acid and sodium thiosulphate react, the solution turns cloudy. You can measure the rate of the reaction by altering the concentration of sodium thiosulphate and measuring the time it takes for the solution to turn fully opaque. The Reaction. When sodium thiosulphate is added to a solution of hydrochloric acid, an insoluble ...
When sodium thiosulphate reacts with hydrochloric acid then it forms the elemental sulphur accompanied by the evolution of sulphur dioxide gas. The reaction that takes place is written as follows: Na2S2O3 (aq) + 2HCL (aq) → → 2NaCl (aq) + SO2 (g) + H2O (l) + S (s) Furthermore, the rate of disappearance or consumption of sodium thiosulphate ...