
- Special Issues
- Conferences
- Turkish Journal of Analysis and Number Theory Home
- Current Issue
- Browse Articles
- Editorial Board
- Abstracting and Indexing
- Aims and Scope
- International Journal of Dental Sciences and Research Home
- Social Science
- Medicine & Healthcare
- Earth & Environmental
- Agriculture & Food Sciences
- Business, Management & Economics
- Biomedical & Life Science
- Mathematics & Physics
- Engineering & Technology
- Materials Science & Metallurgy
- Quick Submission
- Apply for Editorial Position
- Propose a special issue
- Launch a new journal
- Authors & Referees
- Advertisers
- Open Access
- Full-Text PDF
- Full-Text HTML
- Full-Text Epub
- Full-Text XML
- Abdulhameed Alsarraf, Roqaya Alrumaih. Oral Cancer Clinical Presentations – An Illustrative Review. International Journal of Dental Sciences and Research . Vol. 12, No. 2, 2024, pp 19-24. https://pubs.sciepub.com/ijdsr/12/2/1 ">Normal Style
- Alsarraf, Abdulhameed, and Roqaya Alrumaih. 'Oral Cancer Clinical Presentations – An Illustrative Review.' International Journal of Dental Sciences and Research 12.2 (2024): 19-24. ">MLA Style
- Alsarraf, A. , & Alrumaih, R. (2024). Oral Cancer Clinical Presentations – An Illustrative Review. International Journal of Dental Sciences and Research , 12 (2), 19-24. ">APA Style
- Alsarraf, Abdulhameed, and Roqaya Alrumaih. 'Oral Cancer Clinical Presentations – An Illustrative Review.' International Journal of Dental Sciences and Research 12, no. 2 (2024): 19-24. ">Chicago Style

Oral Cancer Clinical Presentations – An Illustrative Review
Oral cancer (OC) is a global health burden with a 5-year survival rate of 50%. It is traditionally defined as oral squamous cell carcinoma due to more than 90% of oral cancers histologically originating in the squamous cells. Early detection of OC improves morbidity accompanying its treatment therefore it is vital for clinicians to recognise the various clinical presentations of OC to facilitate prompt referral and early management. OC has a wide range of presentations with a spectrum ranging from a small asymptomatic lump, red or white, or mixed red and white patch to a large extensive ulcer or growth. In locally advanced cases, pain is usually present accompanied by referred pain to the ear, halitosis, trismus, dysphagia and odynophagia, intra-oral bleeding, weight loss and neck swelling. Due to the wide variation in clinical presentation, general dental practitioners and dental specialists may be unsure of suspicious lesions and the urgency of referrals required in such cases. This review aims to illustrate the clinical presentations of OC using representative clinical photographs from patients attending our Oral Medicine Clinic.
1. Introduction
One of the most significant causes of mortality and morbidity worldwide is cancer 1 . Oral cancer (OC) represents 2% of the cancer burden worldwide with an annual number of new cases exceeding 377,000 and 177,000 mortalities 2 . It most commonly presents as oral squamous cell carcinoma (OSCC) in 90% of the cases 3 . The mucosal lining epithelium is the site at which OSCC originates 3 . OSCC is a tumour involving invasive epithelial cells with a ranging degree of squamous differentiation 4 . OC most commonly presents in males between the ages of 60-79 5 . The incidence of OC has a diverse geographic variation, and a greater burden is observed in low- and middle-income countries such as Latin America, South-East Asia, and Western Europe 6 . This diverse incidence rate seems to be linked to the adaptation of different OC behavioural risk factors 7 .
The modality of management of OC is primarily through surgical resection of the tumour site and may include radiotherapy or chemotherapy 2 . Cancer treatment usually leads to the development of oral symptoms including dysphagia, salivary gland dysfunction, and mucositis 7 . Salivary gland dysfunction may result in altered taste, difficulty swallowing and infections of fungal origin 7 . Patients who undergo radiotherapy or chemotherapy may end up with mucositis which is inflammation of the tissues, resulting in sores and ulcers in 40% of the cases 7 .
Table 1. Anatomical subsites derived from the International Classification of Diseases for Oncology

- Tables index View option Full Size
The management of oral cancer through surgery and radiotherapy may not only result in disfigurement but may also interfere with daily activities such as talking, eating, and drinking 8 . Changes in appearance may follow even with successful reconstructive surgery and patients may end up with an increased risk of airway obstruction, hence may require a tracheostomy and a feeding tube 2 . Anatomical sites in which OC presents are listed in Table 1 . These sites are important for speech, swallowing, and taste, therefore OC and its treatment may have considerable functional sequelae with subsequent impairment of quality of life. This review illustrates a plethora of clinical features of oral cancer presenting in different oral cavity subsites.
2. Clinical Presentations
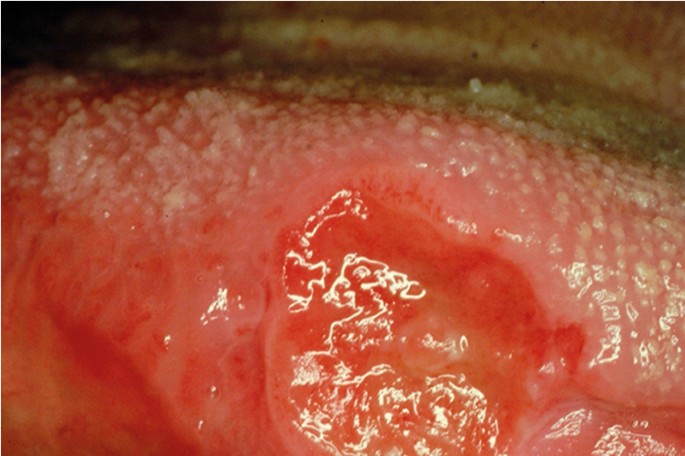
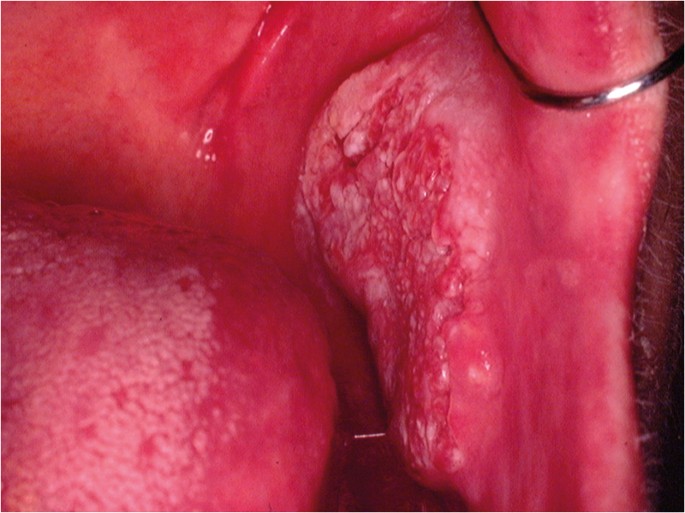
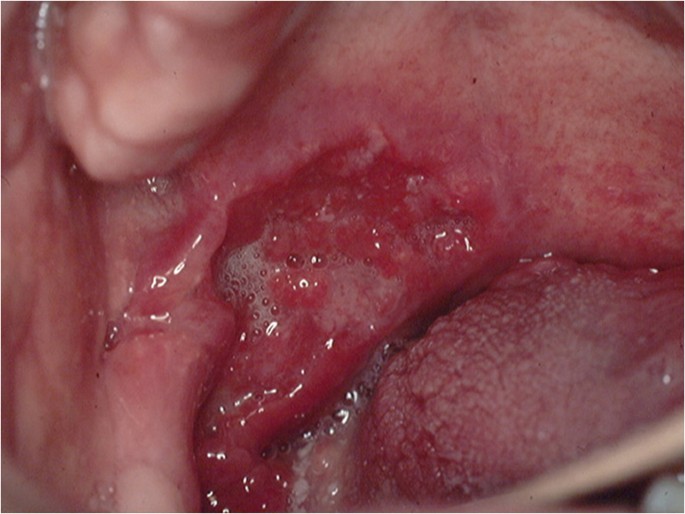
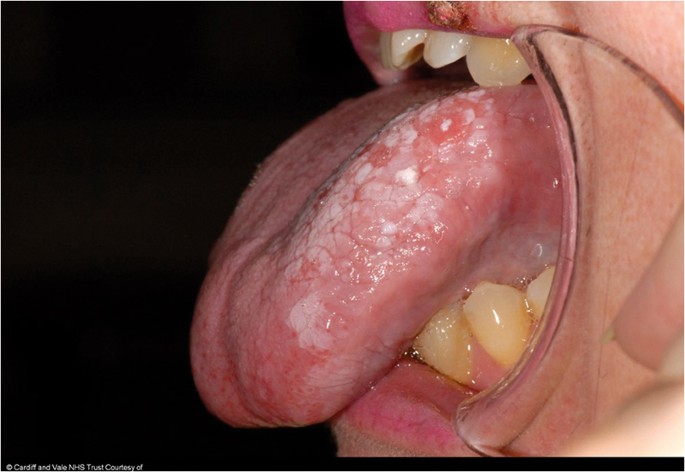
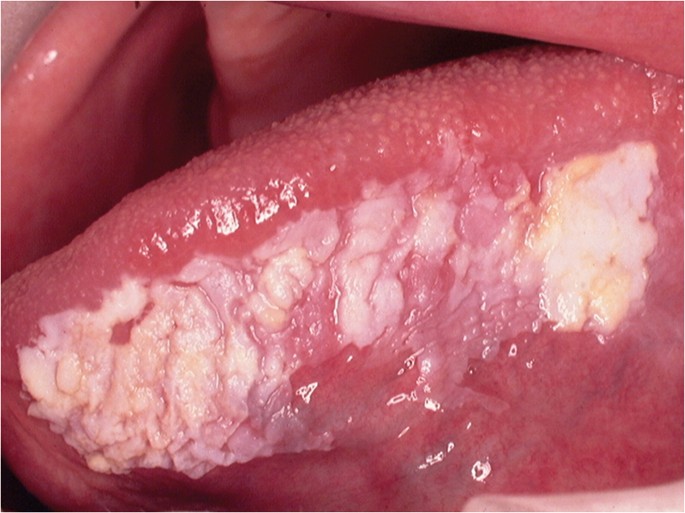
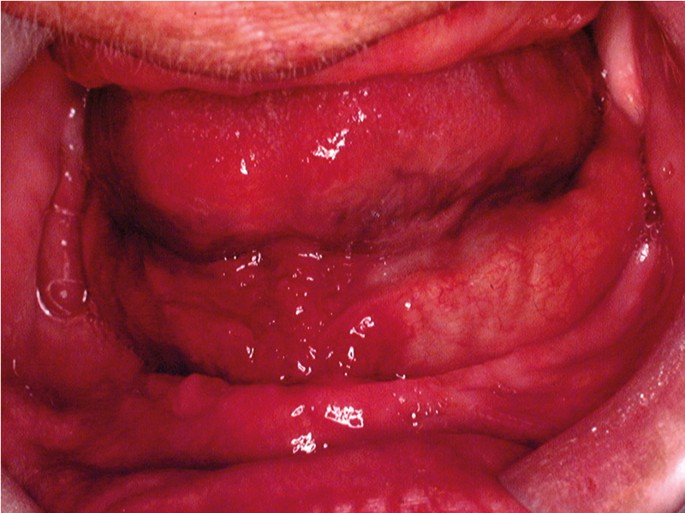
OC may arise in any oral cavity subsite ( Table 1 ). According to the affected subsite, clinical features may vary. OC may be detected at its early stages where lesions may appear as asymptomatic small ulcers or lumps. The size of initial OC lesions can range from a few millimeters to several centimeters as the lesions progress. Lesions may appear as erythroleukoplakias with central ulcerations indicating suspicion for carcinoma in situ or invasive squamous cell carcinoma at the time of detection ( Figure 1 ). Late-stage disease however appears as large growths with rolled margins and surface ulcerations ( Figure 2 ). The tongue is a common oral cavity subsite for the development of OC ( Figure 3 ). Suspicious tongue lesions may present as a small growth-like lesion ( Figure 4 ) to a larger ulcerative lesion extending to the ventral surface ( Figure 5 ). Some tumours present within large non-homogenous leukoplakias ( Figure 6 -8). In advanced cases, tumours present as ulceroproliferative growths with areas of necrosis and extension to surrounding structures such as muscle, bone, and layers of the skin with neck metastasis ( Figure 9 ). The floor of mouth represents the second most common site for the development of OC ( Figure 10 ). Lesions are likely to arise from a pre-existing leukoplakia or erythroplakia ( Figure 11 ). The palate may also be affected with OC when lesions present as non-healing indurated ulcers with a depressed alveolar mucosa ( Figure 12 ). It is worth noting that appropriate retraction of oral tissues aids in the detection of suspicious lumps presenting in edentulous spaces such as the lower posterior alveolar ridge ( Figure 13 ).
3. Risk factors
Risk factors for the development of OC include alcohol abuse, tobacco consumption and betel-quid chewing 10 . Individuals who are heavy smokers and binge drinkers are 38 times more likely to develop OC in comparison to abstainers 6 . This may be due to acetaldehyde, a metabolite of alcohol and a component of tobacco, which is classed as a carcinogen 6 . Acetaldehyde prevents the synthesis of DNA and its repair 2 . Heavy smokers are three times more likely to develop OC than their non-smoking counterparts 2 . Tobacco and alcohol together have a synergistic effect on the risk of developing OC 1 . Smokeless tobacco such as betel quid is sometimes combined with ground areca nut 2 . Areca nut alone without its combination with tobacco is associated with the development of OC as it causes cytotoxic damage of the mucosa through hypoxia and inflammation of the tissues 2 . These behavioural risk factors are thought to cause OC as they interfere with cellular processes and alter genetics 7 . Human Papilloma Virus (HPV) also seems to be linked to the development of OC 10 . A study from 27 countries examining head and neck squamous cell carcinoma revealed a greater risk in those with a low educational background and income regardless of behavioural risk factors such as alcohol consumption and smoking 11 . This shows the independent effect of one’s socioeconomic status on their OC development risk 11 .

- Figure 1. (Tongue) A large erythroleukoplakia with central ulceration (arrow) suspicious for OSCC

- Fig ure 2. (Tongue) An extensive growth-like lesion highly suspicious for OSCC

- Figure 3. (Tongue) A large growth-like lesion with surface ulceration suspicious for OSCC

- Figure 4. (Tongue) Growth-like lesion with a red ulcerative surface suspicious for OSCC

- Figure 5. (Tongue) Large ulcerative lesion extending from the lateral to ventral surface of tongue

- Figure 6. (Tongue) Suspicious tumour in the left aspect (arrow) of the dorsum surface surrounded by an extensive verrucous leukoplakia

- Figure 7. (Tongue) Large erythroleukoplakia suspicious for OSCC

- Figure 8. (Tongue) Highly suspicious growth-like lesion (arrow) presenting superior to a non-homogenous leukoplakia

- Figure 9. (Neck) Metastasis from primary oral cancer

- Figure 10. (Floor of mouth) Suspicious growth-like lesion

- Figure 11. (Floor of mouth) High-risk non-homogenous leukoplakia

- Figure 12. (Palate) A large suspicious ulcer with indurated margins and depressed alveolar mucosa

- Figure 13. (Lower alveolar ridge) A soft tissue lump with irregular surface texture recently developed (arrow), suspicious for carcinoma in situ or OSCC
4. Advanced Stages
In advanced stages of OC, ulceration is one of the most common features presenting with an irregular floor and margins, elevation, and induration. With large lesions, the pain is severe and usually radiates to the ipsilateral ear 12 . Less common presentations include paraesthesia of the chin or delayed healing following a dental extraction or sometimes a lump with abnormal blood vessel supply, dysphagia, or weight loss. In these advanced cases, neck metastases and cervical lymph node enlargement can be seen ( Figure 8 ). Large OC lesions (Stages T3-T4) with lymph node involvement (N2a-N2b) were most associated with mortality in a study on 216 patients over a period of 5 years 13 . Despite advances in medicine, OC has high rates of mortality with a global five-year survival rate of 50% 10 . Treatment leaves patients with high rates of morbidity 14 . In comparison to other cancers such as breast or colon, OC has worse survival rates due to failure of thorough screening, low index of suspicion, and poor dental attendance which all delay the diagnosis 14 . When the diagnosis is delayed, patients present late with more advanced disease which is more challenging to treat and hence a greater negative impact on their quality of life is observed. Cancer survival is heavily dependent on the stage of diagnosis 6 . More than half of patients (60%) with OC present in stages three and four, hence more complex treatment is required 6 . Higher survival rates are seen in patients treated with early stages of OC, hence early disease detection and referral improve survival 5 . A higher incidence of OC is seen in patients with a low socio-economic background and those patients tend to have worse outcomes such as worse survival rates 10 . OC seems to be closely linked to economic and social deprivation, with the highest incidence of disease occurring in those most deprived 6 . Cancer survival trends revealed a gap in survival rates between the most deprived and affluent groups 5 . This may be due to several factors including barriers to access and inadequate awareness 1 .
5. Clinical Significance
At the time of diagnosis, approximately half of OC patients present with metastases either regional or distant, leading to greater rates of mortality 15 . Late presentation is also associated with more complex treatment of an increased cost and increased morbidity 3 . In order to detect disease at early stages, a comprehensive oral examination should be routinely carried out by clinicians and if a potentially malignant lesion is present, referral to a specialist is required for a definitive diagnosis 14 . Malignant transformation is determined after histopathological grading of a specimen is completed to determine the presence and extent of oral epithelial dysplasia 15 . OSCC is usually preceded by oral potentially malignant disorders such as leukoplakia, erythroplakia and submucous fibrosis 16 . On some occasions, OSCC may develop from sound epithelium free of dysplastic changes 14 . However, a visible pre-clinical phase of dysplasia precedes most OSCC cases 17 . Screening could prevent malignant changes or ensure that disease is diagnosed at earlier stages 18 . It is the process of identifying individuals at increased risk of a disease from an apparently healthy population 18 . Screening is seen in multiple forms such as screening an entire population, or selectively where the focus is only on high-risk individuals, or opportunistically when individuals are screened after attending for other reasons 6 . OC screening is a straightforward, quick, cheap procedure that involves good lighting, gloves, and gauze in comparison to breast cancer screening which involves mammograms 3 . The incidence and mortality rates have been significantly improved after screening programmes have been introduced for common cancers such as breast and bowel cancer 6 . Screening programmes may allow for early disease detection and improved survival 3 . Clinicians are also encouraged to keep a detailed record of a lesion including the site, size, texture, borders, colour, and clinical photography is advised to monitor the lesion.
Guidelines on practices and treatment enhance outcomes as they set the standards for managing patients 1 . It is encouraged that local guidelines are developed for the referral of patients with potentially malignant lesions. In the United Kingdom, a 2 week wait pathways has been developed to ensure patients with a red/white patch, ulcer, or a new growth present in the mouth for more than three weeks are referred urgently to specialist services 14 . Having a clear referral system reduces error and ensures that patients are seen in a timely manner. Developing national guidelines may bridge the gap between general practitioners and specialists, hence minimising abuse of the system with unnecessary referrals and maximising effectiveness.
It is important to emphasise the crucial role of disease prevention in which General Dental Practitioners (GDP) identify risk factors through history taking and address them appropriately as part of the holistic treatment plan. Cultural barriers should be overcome especially when taking a social history. GDPs are expected to give smoking cessation and alcohol advice or signpost patients to the appropriate services. Managing those risk factors will not only reduce the risk of OC, but of other diseases as well. HPV vaccinations have been introduced globally. Patients should be encouraged to take the vaccine at appropriate ages. Governments tend to place resources on cancer treatment with a low emphasis on prevention 1 . According to the World health Organization, it is advised that measures are put in place for prevention of OC as part of local cancer control programmes 3 . Hence campaigns should be developed to shed light on risk factors, signs, and symptoms of OC. Policy makers could work on initiatives to address risk factors such as taxing tobacco.
Additionally, the awareness of General Medical Practitioners and medical disciplines including otolaryngologists, gastroenterologists, and dermatologists with regards to OC is important. Many patients with oral diseases present to otolaryngology departments since patients consider the oral cavity to be within the realm of the throat, while many gastroenterologists assume oral lesions develop due to gastrointestinal disease 19 . The ultimate aim is to enhance early detection thereby improving prognosis for patients presenting with OC.
6. Conclusion
Oral cancer is a preventable disease and early detection can reduce cancerous transformation hence potentially improve survival rates. General dental practitioners and medical disciplines have an important role in giving advice on risk factors implicated in OC and to recognise and promptly refer suspicious lesions. This review illustrates a plethora of clinical features of OC presenting in different oral cavity subsites. Knowledge of the various clinical features facilitates prompt referral to specialist services for diagnosis and management.
ACKNOWLEDGEMENTS
The authors wish to thank the patients for giving consent to obtain clinical photographs.
Published with license by Science and Education Publishing, Copyright © 2024 Abdulhameed Alsarraf and Roqaya Alrumaih
Cite this article:
Normal style, chicago style.
- Google-plus

- View in article Full Size Figure
- View in article Full Size
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 09 November 2018
Mouth cancer: presentation, detection and referral in primary dental care
- M. A. O. Lewis 1
British Dental Journal volume 225 , pages 833–840 ( 2018 ) Cite this article
4392 Accesses
12 Citations
22 Altmetric
Metrics details
Discusses the highly variable way in which mouth cancer can present clinically and as such any persistent mucosal abnormality should be viewed with suspicion.
Emphasises that the detection of mouth cancer while the tumour is less than 2 cm in diameter is the single most important factor that can improve patient outcome.
Provides information on diagnostic aids for mouth cancer and potentially malignant mucosal disorders.
Outlines the guidance on the appropriate referral of urgent suspected cancer.
Mouth cancer can present as a variety of abnormalities and visible changes affecting the oral mucosa, including ulceration, swelling and areas of erythema. The five-year survival from mouth cancer is poor at approximately 50%. Detection of the cancer while less than 2 cm in diameter with no metastasis greatly improves the outcome for the patient. Although many cancers in the mouth develop from what was previously an apparently normal mucosa, some arise in pre-existing conditions that are therefore regarded as potentially malignant. Regular assessment of the soft tissues within the mouth and the neck for the presence of abnormalities is an essential component of primary dental care. Any persistent and unexplained abnormality requires referral for definitive diagnosis and specialist management.
You have full access to this article via your institution.
Introduction
Certain conditions that affect the oral mucosa have characteristic clinical signs and symptoms that allow diagnosis on the basis of clinical presentation alone without the need for special investigations. Examples of these would be the distinctive history and appearance of minor recurrent aphthous stomatitis and geographic tongue. However, squamous cell carcinoma (mouth cancer) contrasts with this markedly in that it can present with a range of mucosal changes and symptoms. On this basis any persistent solitary mucosal abnormality should be regarded as potentially sinister until proven otherwise.
One of the main reasons that that mouth cancer is given such high importance in dentistry and has been recommended as a topic for continuing professional development is the poor five-year survival, which overall is approximately 55%. 1 The single most important factor that can improve five-year survival is detection of the tumour whilst small, specifically 2 cm or less in diameter with no regional node involvement or distant metastases (Stage I). Patients with a tumour detected at Stage I are associated with an 85% five-year survival compared to those with Stage IV (greater than 4 cm in diameter with regional node involvement and possible distant metastasis) for whom the five-year survival is only 10%. 1 , 2 The importance of detection while small was also highlighted in a recent retrospective study of survival after aggressive surgery which revealed that a patient with a tumour that is detected with a maximum diameter 2 cm (tumour stage T1) had a mean post-treatment survival of 24.48 years (95% CI 22.45–26.50) while a patient with a tumour greater than 4 cm (tumour stage T4) had a mean survival of only 13.03 years (95% CI 11.56–14.49). 3 These figures for survival are longer than those reported in studies and in part it must be appreciated that the research involved only one centre. In reality the five-year survival from advanced cancer is usually much shorter than 13 years.
It is important to appreciate that velocity of cancer growth in the mouth is not uniform. Some tumours may slowly increase in size over many months while others enlarge rapidly over a few weeks. On this basis the concept of 'early' diagnosis is slightly misleading since it is not actually the time that the cancer has been present that is most important but the size and presence of regional metastasis when the tumour is first detected that is key to an improved chance of survival for the patient.
It is useful to recognise that oral squamous cell carcinoma essentially represents epithelial cell replication within the lining of the mouth that is 'out of control' and as such is a surface event which should be visible on clinical examination. This feature contrasts with some other tumours that are not 'visible', such as ovarian cancer or pancreatic cancer. It is not unreasonable to expect a dental care professional to be able to detect a surface mucosal abnormality that is 2 cm in diameter since this size is approximately that of a human finger nail.
Clinical presentation
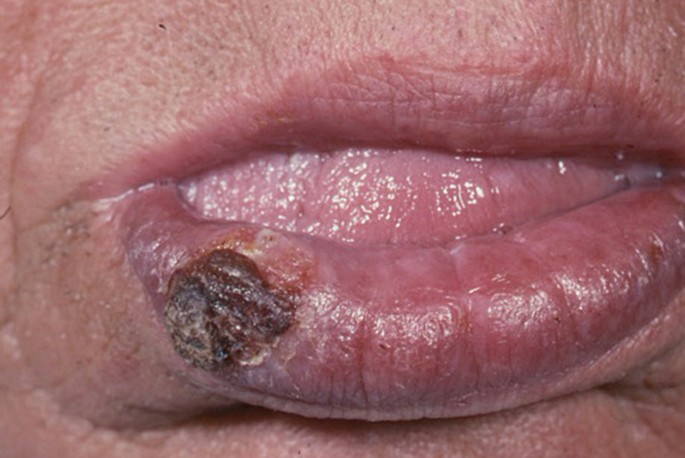
The classical appearance of mouth cancer is a solitary deep ulcer with rolled margins on the lateral margin of the tongue ( Fig. 1 ). Palpation of the margin of the ulcer will reveal firm, often described as indurated, tissues. Unfortunately however, other than induration, mouth cancer has no pathognomonic presenting feature and involves a spectrum of mucosal changes in addition to ulceration including swelling ( Fig. 2 ), erythema ( Fig. 3 ) or a speckled red/white patch ( Fig. 4 ). 4
Squamous cell carcinoma (SCC) as a solitary deep ulcer with rolled margins with no obvious cause
SCC as mucosal swelling with speckled surface in left buccal mucosa
SCC as an erythematous mucosal patch in left oropharynx
SCC as a predominantly white mucosal patch on the left lateral margin of the tongue
Examination of the patient
A number of educational videos showing methods of clinical examination of the mouth and neck are freely available on the Internet via providers such as YouTube or Google video. However, the three minute 'Oral Cancer Recognition Toolkit' video developed by Cancer Research UK and the British Dental Association is particularly useful for dental professionals. 5
Extra-oral examination
The soft tissues of the neck should be palpated on both sides using the tips of the fingers to detect any abnormal tissue swelling or enlargement of the lymph nodes. The patient's neck should be flexed and the examination start in the submental region moving back to the submandibular region, then down the jugular chain to the supra-clavicular fossa.
Intra-oral examination
There is no correct order for examining the oral soft tissues as long as that at the completion of the examination the entire mouth and the oropharynx, including the tonsils has been assessed. Good lighting is obviously essential. Any mucosal abnormality noted should be palpated to determine consistency of the soft tissue ( Fig. 5 ). As a general rule, induration reflects the presence of a benign or malignant neoplasm while softness to palpation represents a non-neoplastic inflammatory condition. In addition, mouth cancer is often painless until well advanced while inflammatory conditions are usually painful from the outset.
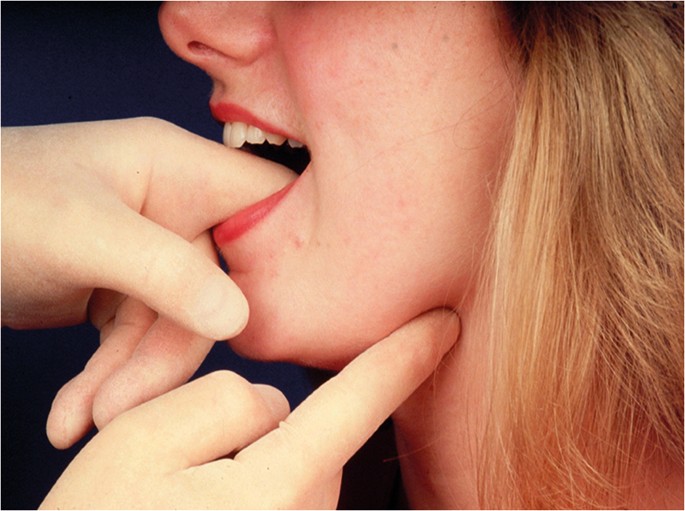
Bimanual palpation of soft tissues in the floor of mouth
Mucosal biopsy
A scalpel biopsy is the gold standard investigation for the diagnosis of any mucosal abnormality. The biopsy may be excisional or incisional depending on the size of the abnormality detected. Small areas of mucosal change can be removed with a surrounding margin of clinically normal mucosa. There is divided opinion on the complete removal of a small mucosal abnormality that is found to be cancer since it may subsequently cause problems in identifying the primary site. However, the vast majority of small localised mucosa lesions are not squamous cell carcinoma and in the cases where they are modern imaging techniques and clinical examination will indicate the diagnostic surgical site. In the case of larger areas of change, a clinical decision has to be made as to what tissue to remove. As a rule the most suspicious looking area of mucosa should be included in the biopsy material. The tissue specimen should therefore include any aspects of ulceration, induration, erosion and erythema along with some apparently normal adjacent mucosa.
Following the placement of local anaesthesia, an incisional mucosal biopsy should involve the taking of an ellipse of tissue from the affected site. It is helpful to firstly place a suture in the tissue to be removed. 6 This is preferable to the use of toothed-tissue tweezers since these may become dislodged and frequent re-application can cause crush damage to the biopsy material. A number-15 blade with a rounded tip is the scalpel of choice for the majority forms of oral surgery. The lower incision is made first to prevent bleeding potentially obscuring the site of the upper incision ( Fig. 6 ). The tissue can then be removed as a sheet of mucosa. Closure of the surgical defect can be made with simple interrupted sutures using either a resorbable or non-resorbable material ( Fig. 7 ). A wide range of suture materials are available and their use depends of the type of surgery being undertaken. Although black silk, a non-resorbable material, has historically been used for intra-oral soft tissue closure, resorbable polyglactin 910 (a copolymer of 90% glycolide and 10% L-lactide), is now the preferred material. Sutures are available on a variety of needles which differ in cross section, size and length. A 19 mm curved reverse cutting needle, which is triangular in cross section, is the needle of choice for closure of the oral mucosa.
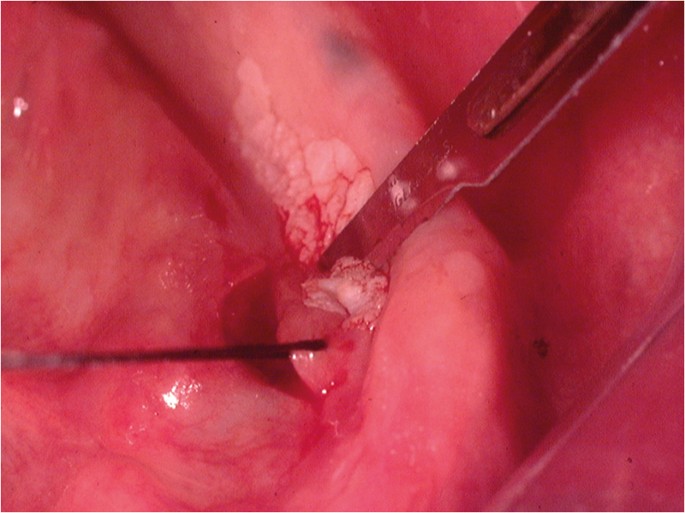
Lower incision during an incisional biopsy of white patch in the floor of the mouth
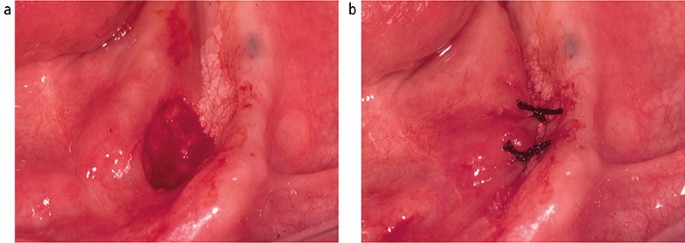
Closure of biopsy defect using simple interrupted silk sutures (b)
The biopsy tissue removed can be supported on filter paper before placement in a pre-labelled specimen pot containing 10% neutral buffered formalin to minimise the impact of shrinkage and distortion during fixation ( Fig. 8 ). This step is important since curling up of the tissue can cause cross sectioning that may be mistaken for epithelial invasion in tissue sections when examined microscopically in two dimensions.
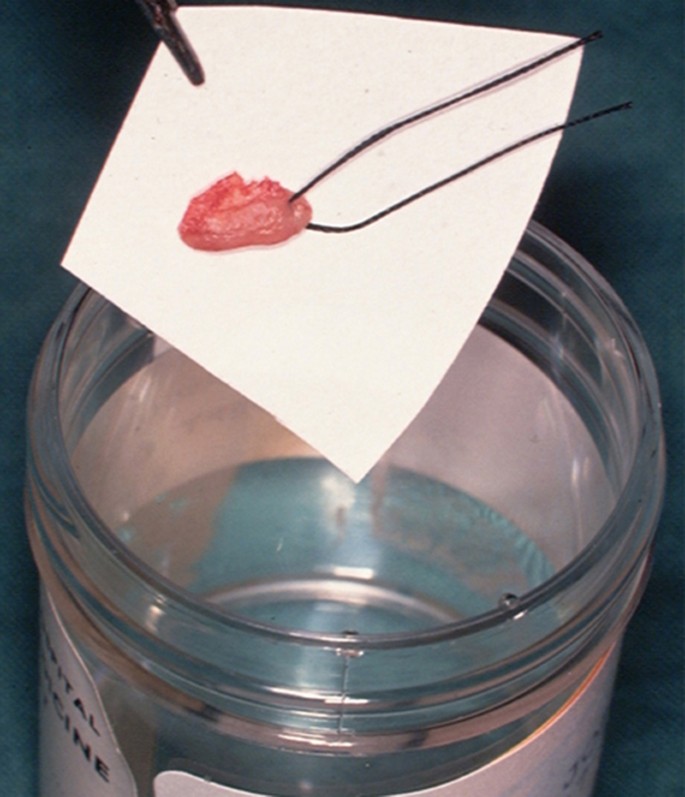
Biopsy tissue placed on filter paper before placement into pathology specimen pot
Punch biopsy
Punch biopsy, which removes a cylindrical specimen of tissue of between 0.4–0.8 mm in diameter, has been promoted as a simple and quick method of sampling the oral mucosa. 7 However, since the amount of tissue removed is relatively small there is potential to not obtain sufficient material when assessing the epithelium for the presence of dysplasia or invasion. On this basis some oral and maxillofacial pathologists have expressed a preference for a scalpel biopsy rather than punch biopsy (personal communication).
Potentially malignant conditions
A primary mouth cancer can either arise in what was previously clinically normal tissue or develop within a pre-existing mucosal abnormally. Such pre-existing conditions that are associated with an increased incidence of undergoing malignant transformation are referred to as being oral potentially malignant disorders (OPMDs) ( Box 1 ).
Leukoplakia
The most frequently recognised OPMD is leukoplakia ( Fig. 9 ), which was first defined by the World Health Organisation (WHO) in 1978 as 'a white patch or plaque that cannot be characterised clinically or pathologically as any other disease'. 8 The term has subsequently been refined following various international workshops and is now used to describe 'white plaques of questionable risk having excluded (other) known diseases and disorders that carry no increased risk for cancer'. 9 It is essential to remember that leukoplakia is a clinical term and not a diagnosis. A biopsy is required to exclude known mucosal disorders. Although often associated with the presence of epithelial dysplasia, leukoplakia itself has no specific histopathological features. The reported malignant transformation rate for oral leukoplakia has ranged from low levels of 0.13% in India 10 and 2.6% in the UK 11 to high levels of 17.5% in USA. 12 These findings are undoubtedly influenced by the geographical site, population studied, number of patients and length of follow-up observation period. The global malignant transformation rate of leukoplakia is generally accepted to be 1.36% per year. 13
Leukoplakia on the left lateral margin of the tongue
There is a rare form of leukoplakia, termed proliferative verrucous carcinoma (PVL), which has a reported transformation rate as high as 70%. 14 The presentation of PVL is characterised by an initial white patch that develops into multiple areas of exophytic/wart-like changes within the mucosa. The aetiology of PVL is unknown and treatment difficult due to progressive recurrence following surgical removal.
Erythroplakia
Oral erythroplakia ( Fig. 10 ) is defined as 'a fiery red patch that cannot be characterised clinically or pathologically as any other definable lesion'. 15 Erythroplakia, in a similar way to leukoplakia has no specific histopatholigical features. The term is used clinically to record the presence of an erythematous mucosal abnormality that does not have a clinical appearance characteristic of known red patches, such as denture-associated candidosis or median rhomboid glossitis. Oral erythroplakia has the highest transformation rate of all of the OPMDs, being reported as between 14–50%. 16 Erythroleukoplakia is an alternative clinical term that can be used when the mucosa has a speckled red and white appearance. Importantly erythroleukoplakia is associated with a high risk of malignant change.
Erythroplakia in the right side of the floor of mouth
Lichen planus
The aetiology of oral lichen planus (OLP) is unknown but does involve immune system since a primary histopathological feature is a sub-epithelial band of T-lymphocytes, indicating a cell-mediated reaction. OLP is one of the most frequently occurring mucosal conditions in the population, with a reported prevalence of between 0.5–2.2%. 17 Different types of OLP have been described, including reticular, atrophic, erosive, plaque-like and bullous, based on the appearance of the mucosa. However, actual typing in an individual patient is often difficult since different types may be present simultaneously and also change during the course of disease over months. Overall, the most characteristic feature is the presence of bilateral white striations in the buccal mucosa ( Fig. 11 ). The reported malignant transformation for OLP worldwide has varied between 0.4–6.4% depending on population studied and length of follow-up period. Two historical UK-based studies revealed yearly transformation rates of 0.07% and 0.27%. 18 , 19 A recent systematic review revealed an increased transformation risk in females with red clinical forms on the tongue. 20
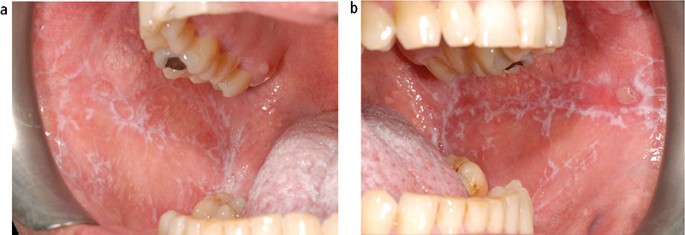
Lichen planus presenting as bilateral and symmetrical white striations in the buccal mucosa; (a) left and (b) right
Submucous fibrosis
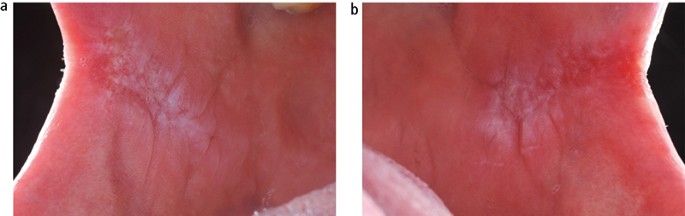
Submucous fibrosis is a chronic disorder of the upper alimentary tract, which presents most obviously within the mouth as vertical fibrous bands in the buccal mucosa that limit mouth opening. The patient will also complain of an overall burning sensation within the mouth, and the buccal mucosa may appear white or erythematous ( Fig. 12 ). Oral submucous fibrosis (OSF) has a strong association with the social habit of chewing areca (betel) nut, which is popular in populations living in and originating from the Indian subcontinent and surrounding countries. Alkaloids within the nut stimulate fibroblast proliferation. The malignant transformation rate from a long-term, follow-up study of OSF in an Indian population was reported as 7.6%. 21
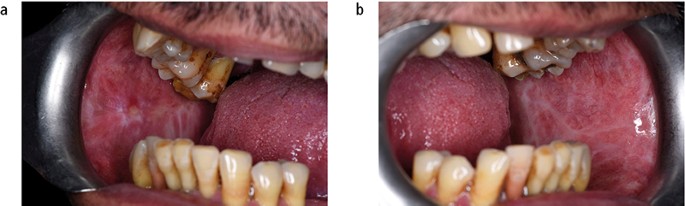
Submucous fibrosis presenting as bilateral white patches in the buccal mucosa; (a) left and (b) right
Palatal keratosis in reverse smokers
Reverse smoking, in which the lit end of a cigar or cigarette is placed in the mouth, is popular in the rural populations of the Amazon, New Guinea and Indian subcontinent. The physical irritation from heat and tobacco smoke induces hyper-keratinisation and erythamatous changes within palatal mucosa. A high incidence of cancer in the hard palate, which is a relatively rare site in non-reverse smokers, has been associated with this habit. 22
Other OPMDs
Actinic keratosis is associated with exposure to ultraviolet light and characteristically affects the vermilion lower lip presenting as palpable white plaques. Regular review is required and the development of palpable induration would indicate the need for biopsy to exclude transformation into either a squamous cell carcinoma or basal cell carcinoma ( Fig. 13 ). The actual transformation rate of actinic keratosis is unknown. Discoid lupus erythematous, which can on occasion produce oral signs that resemble oral lichen planus or erythroplakia, has been reported to transform into squamous cell carcinoma. Dyskeratosis congenita, an example of inherited OMPD, is a bone marrow disorder that is associated with oral white patches ( Fig. 14 ) which transform into mouth cancer and cause death in young adulthood. 23
Squamous cell carcinoma arising in pre-existing actinic keratosis
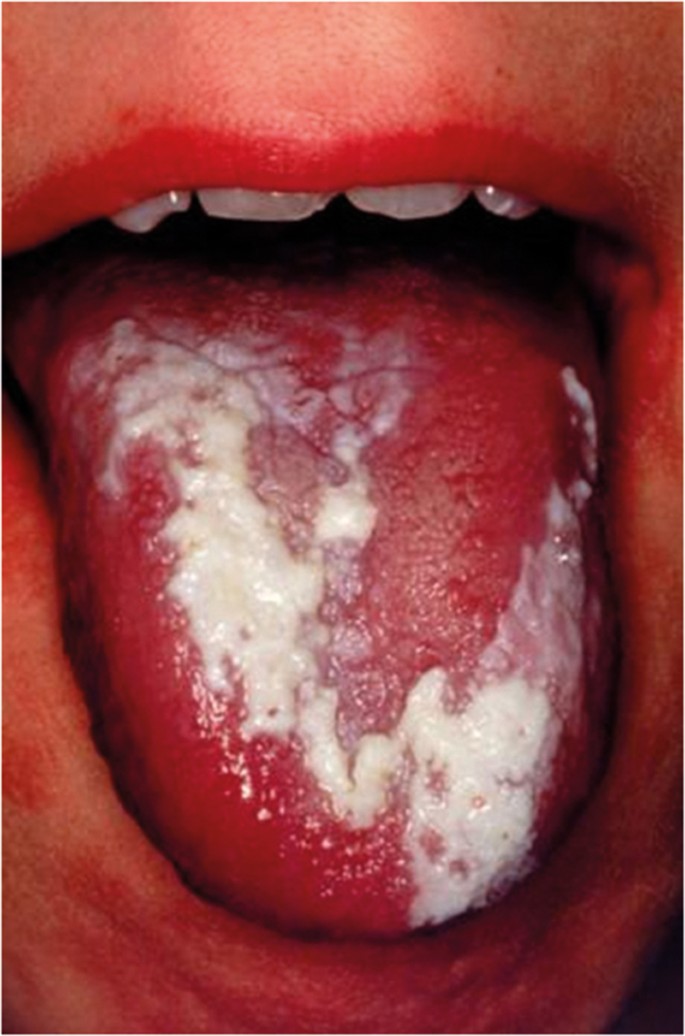
White patch on dorsal surface of the tongue in dyskeratosis congenital (Courtesy of Professor Graham Ogden)
Although not included in the WHO list of OPMDs, chronic hyperplastic candidosis (CHC) is recognised as having the potential to undergo malignant transformation. 24 CHC characteristically presents as bilateral adherent white patches in the buccal commissure regions of the mouth ( Fig. 15 ) or dorsum of tongue. This type of oral candidosis is seen almost exclusively in smokers. Although the provision of systemic antifungal therapy will produce a dramatic clinical improvement, CHC will recur if the patient does not stop the tobacco habit. All patients with CHC should be provided with smoking cessation advice.
Chronic hyperplastic candidosis presenting as bilateral white patches in the buccal mucosa; (a) left and (b) right
Box 1: Oral potentially malignant disorders of the oral mucosa 9
Palatal lesions in reverse smokers
Oral submucous fibrosis
Actinic keratosis
Discoid lupus erythematosus
Detection aids
As mentioned above, mouth cancer has a wide spectrum of clinical presentation with no pathognomonic feature. It has been demonstrated that dentists, dental hygienists and dental therapists are able to confidently recognise mucosal abnormalities and have relatively high accuracy for the clinical detection of mouth cancer or OMPD. 25 However, it is generally accepted that even while the most experienced clinician may have strong suspicion of the presence of squamous cell carcinoma, there can never be 100% certainty until the diagnosis is confirmed on the basis of histopathological findings following gold standard mucosal biopsy. Occasionally a highly suspicious clinical area of mucosa change is found to be an essentially benign abnormality and conversely a benign-looking mucosal abnormality can be found to contain a carcinoma. The lack of specific clinical signs for mouth cancer probably in part accounts for the delay in a patient seeking an opinion of dentist or doctor in primary care and hence the high percentage of patients who are not diagnosed till the tumour has advanced to Stage III or Stage IV. 1
A range of adjunctive clinical aids have been and are continuing to be developed commercially to increase the detection of potential mouth cancer at the chair side. 26 The technology involved includes the use of nuclear dyes, tissue reflectance light visualisation and fluorescence imaging, all of which aim to increase the clinician's ability to detect an area of mucosal abnormality that may represent cancer or OMPDs. The primary aim of such diagnostic adjuncts is to increase the proportion of mouth cancers that are detected in primary care while at Stage I or Stage II. The promotion of these diagnostic aids not only emphasises the importance within the dental profession to thoroughly assess the oral mucosa but when used in the clinic also raises awareness of the potential of mouth cancer in patients. However, the subjective interpretation of the tests coupled with relatively good sensitivity but poor specificity, makes their use problematic. There is limited evidence to support the use of light-based techniques in primary care. 27 , 28 At the present time further research is required to develop the technology to achieve a tool that could be used as an effective screen for mouth cancer within the general population. It is not possible to present details of all the available diagnostic adjuncts here (for reviews, see Macey et al . 29 and Lingen et al . 30 ). Some of the more widely known systems are described below.
Vital tissue staining
The use of vital tissue staining in cancer detection is based on the assumption that a nuclear dye will be taken up in the epithelial cells where there is a high density of nuclear material, such as in malignancy, and therefore have a different appearance to normal mucosa. This methodology was originally used for cervical screening in women. Toluidine blue (tolonium chloride) is a metachromatic dye has been used widely in this context. Retention of the dye, seen as dark blue, following application and de-staining with acetic acid is considered positive ( Fig. 16 ). However, it is not cancer specific and false positives due to uptake by benign inflammatory and ulcerative of the oral mucosa are frequent. Equally, failure of the dye to penetrate keratinised mucosa can lead to false negatives. A Cochrane review of the use of the toluidine blue test for detection of oral carcinoma or OPMDs in 14 studies reported an overall sensitivity of 0.84 and specificity of 0.70 for both conditions. 29
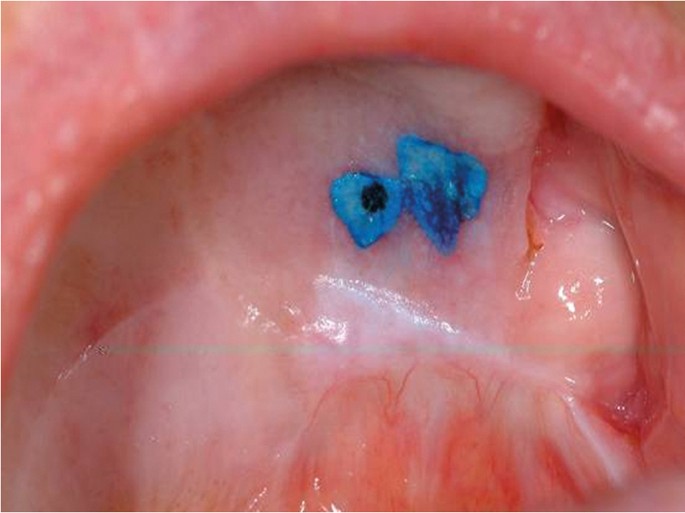
Toludine blue stain retained on palatal mucosal abnormality
Tissue reflectance light visualisation
The use of tissue reflectance or chemiluminescence to detect cancer is based on the theory that the increased nuclear to cytoplasmic ratio of malignant epithelial cells results in increased light reflectance when compared to normal mucosa. ViziLite Plus (Panadent, Orpington, UK) and MicroLux DL (Panadent, Orpington, UK) are two cancer detection systems that employ this approach. The systems differ in their light sources with ViziLite Plus using a disposable stick (490–510 nm wavelengths) and MicroLux DL using a battery operated device (410–710 nm). Abnormal mucosa appears 'aceto-white' when compared to normal tissues which are light blue. Both systems have been found to brighten areas of leukoplakia, which had already been detected using conventional examination. Unfortunately, due to the lack of surgical biopsy information in the studies evaluating these two systems it is not possible to determine sensitivity and specificity figures.
Fluorescence imaging
It has been discovered that cancer cells have different autofluorescence emission patterns when compared to normal tissues. The Visually Enhanced Lesion Scope (VELscope, LED Dental Inc, White Rock, British Columbia, Canada) is a handheld device that shines high-intensity blue excitation light (400–460 nm wavelengths) onto the oral mucosa. Normal tissues fluoresce green while malignant cells appear relatively dark. Unfortunately, some normal tissue or benign conditions may also remain dark. A Cochrane review of twelve studies of the use of VELscope reported an overall sensitivity of 0.91 and specificity of 0.58 for oral carcinoma or OPMDs. 29
Brush cytology
Exfoliative cytology has been investigated as a possible mouth cancer detection system. OralCDx (OralCDx Laboratories, Inc) is an example of this technology that uses a special designed brush to collect cells from an identified mucosal abnormality within the mouth. The sample is then sent for computerised analysis for the presence of abnormal cells and reported as negative, atypical or positive. All atypical and positive results require a tissue biopsy. OralCDx has been found to have a sensitivity of 0.92 and specificity of 0.94 to detect dysplasia or squamous cell carcinoma. 31 More recently cytological methods have been combined with molecular markers in an attempt to improve their prognostic value.
Salivary diagnostics
Molecular biology has enabled the ability to detect biomarkers (peptides, proteins, DNA, mRNAs and miRNAs) in saliva and as such provide a non-invasive diagnostic test and screening tool for human disease. The potential to use saliva to detect the presence of cancer has been researched in relation to tumours of the ovary, breast, pancreas and lung. In addition, a number of studies have been undertaken to look at biomarkers for oral squamous cell carcinoma. 32 , 33 , 34 , 35 At the present time further work is required to identify specific biomarkers within saliva that can be reliably used to detect different forms of human cancer and other forms of disease. Salivary diagnostic technology has huge potential for the future and could become a routine aspect of dental primary care.
As previously described, the outcome of mouth cancer is significantly improved if the tumour is detected and treated when it is less than 2 cm in diameter with no local metastasis. On this basis any suspected cancer patient should be referred for specialist assessment rapidly. Terminology used in this situation is urgent suspected cancer (USC) with an expectation, in the UK, that a patient referred a USC will be seen within 14 days (2 week wait, 2WW) or 10 working days(10 day rule). 1 The 2WW system for cancer, including head and neck cancer, was introduced in the UK in 2000 and there is little doubt that it has been valued by patients since it speeds up the overall management. An audit of 2WW rule in the oral and maxillofacial surgery department at the Newcastle General Hospital and then subsequently in the oral surgery and oral medicine departments at Newcastle Dental Hospital revealed positive head and neck cancer detection rates of 11% and 7% respectively in the cohorts of patients examined. 36 A similar study at the oral medicine department in King's College, London, reported that 8% of urgent referrals were found to have oral cancer. 37 Both audits concluded that further education of referring practitioners and refinement of the referral guidelines were required to ensure a more efficient service.
A systematic review of the impact of the 2WW rule for head and neck cancer between 2000–14 concluded that the conversion rate (proportion of 2WW referrals who were diagnosed as having cancer) was falling, and the detection rate (proportion of diagnosed cancers referred under 2WW rule) was rising due to increased number of referrals. In addition the impact of the 2WW rule on outcome, particularly survival, was not clear. 38
Methods of referral from primary care to specialist secondary care vary widely. Historically, referrals have been made in the form of a written letter with inevitable risk of delay within a postal system. This has in part been overcome by the use of a fax machines or a secure NHS email address. Personal email accounts must not be used due to the high risk of breach of patient confidentiality. However, free written communication via any of these methods may be problematic in that the clinical information provided by the primary care practitioner may be insufficient to enable the receiving specialist to identify the need for a 2WW appointment. The inclusion of a question on a preformed referral forms 'Do you think that this patient may have a cancer?' or a 'Yes/No' tick-box for USC is helpful but again not foolproof. Details of risk factors for the patient, in particular any tobacco, betel nut or alcohol habits, are important to include.
Electronic record management and referral systems within managed clinical networks (MCNs) are being introduced and these have a range of advantages, including an assurance of rapid and safe delivery of the referral request. In addition, some electronic systems ensure that all relevant information is provided by the referring practitioner since the referral will not be accepted until this is completed online. Individual practitioners must be aware of their local referral system.
In the UK, the National Institute for Health and Clinical Excellence (NICE) 39 and Healthcare Improvement Scotland (HIS) 40 have published referral guidelines to help clinicians decide which patients should be refereed as USC. These guidelines focus on the presenting clinical symptoms and recommend the need for a USC referral for any individual with:
Unexplained ulceration in the oral cavity lasting more than three weeks
A persistent and unexplained lump in the neck
A lump on the lip or in the oral cavity consistent with oral cancer
A red or red and white patch in the oral cavity consistent erythroplakia or erythroleukoplakia.
There has been discussion in relation to the recommendation that a doctor should refer a patient to a dentist for assessment within two weeks if the doctor thinks that there is 'a lump on the lip or in the oral cavity consistent with oral cancer or a red or red and white patch in the oral cavity consistent erythroplakia or erythroleukoplakia'. Concern has been expressed that due to the lack of a clear pathway between the doctor and the dentist this advice exposes the patient to unnecessary delay in diagnosis. 41 , 42
Communication with the patient
If a soft tissue examination reveals an abnormality and it is felt that a referral is indicated then what should the patient be told? It is probably best to inform the patient that changes in the mouth are seen frequently and the vast majority are innocent but it is best to get it double checked by a specialist. Other information given to the patient and/or carer could include:
Where there are being referred
How long they may have to wait
Who will see them
What types of test may be done. 43
This communication has to be done sensitively and obviously the amount of information given decided on an individual patient basis.
Interestingly, it has been reported that some dentists have a reluctance to discuss mouth cancer with their patients due to a lack of confidence to answer patients' questions. 44 There would appear to be a need for specific training and guidance for the dental team on how to raise the issue of mouth cancer during routine examination and also how to communicate any issues without causing unnecessary anxiety. This aspect of mouth cancer could be a subject for future undergraduate and postgraduate education.
The clinical presentation of mouth cancer is highly variable. Regular and thorough examination of the soft tissues to detect any abnormality is an essential aspect of dental primary care. If any mucosal change found is thought to possibly represent cancer, then the patient needs to be referred appropriately for a specialist evaluation. All dental professionals should be aware of mouth cancer and feel comfortable about discussing the subject with their patients.
Cancer Research UK Website. Available at www.cancerresearchuk.org/health (accessed August 2018).
Sciubba J J . Oral cancer. The importance of early diagnosis and treatment, Am J Clin Dermatol 2001; 2 : 239–251.
Article Google Scholar
Ong T K, Murphy C, Smith A B, Kanatas A N, Mitchell D A . Survival after surgery for oral cancer: a 30-year experience. Br J Oral Maxillofac Surg 2017; 55 : 911–916.
Lewis M A O, Lamey P-J . A clinical guide to oral medicine . 3rd ed. London: British Dental Journal, 2011.
Google Scholar
Oral cancer recognition toolkit. Available at www.doctors.net.uk/eclientopen/cruk/oral_cancer_toolkit_2015_open/ (accessed 19 October 2018).
Oliver R J, Sloan P, Pemberton M N . Oral biopsies: methods and applications. Br Dent J 2004; 196 : 329–333.
Lynch D P, Morris F M . The oral mucosa punch biopsy. J Am Dent Assoc 1990; 121 : 145–149.
Kramer I R H, Lucas R B, Pindborg J J, World Health Organization Collaborating Centre for Oral Precancerous Lesions: Definition of leukoplakia and related lesions. An aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol 1978; 46 : 518–539.
Warnakulasuriya S, Johnson N W, van der Waal I . Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007; 36 : 575–580.
Silverman S, Bhargava, K, Smith L W, Malaowalla A M . Malignant transformation and natural history of oral leukoplakia in 57: 518 industrial workers of Gujarat, India. Cancer 1976; 38 : 1790–1795.
Warnakulasuriya S, Kovacevic T, Madden P et al. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10-year period in South East England. J Oral Pathol Med 2011; 40 : 677–683.
Silverman S, Gorsky M, Lozada F . Oral Leukoplakia and malignant transformation: a follow up study of 257 patients. Cancer 1984; 53 : 563–568.
Petti S . Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncol 2003; 39 : 770–780.
Munde A, Karle R . Proliferative verrucous leukoplakia: An update. J Can Res Ther 2016; 12 : 469–473.
Pinborg J J, Reichart Smith C J, van der Waal I, Sobin L H and pathologists in nine countries. World Health Organization international histological classification of tumours, histological typing of cancer and precancer of the oral mucosa. 2nd ed. Berlin: Springer, 1997.
Reichart P A, Philipsen H P . Oral erythroplakia – a review. Oral Oncol 2005; 41 : 551–561.
De Rossi S S, Ciarrocca K . Oral lichen planus and lichenoid mucositis. Dent Clin North Am 2014; 58 : 299–313.
Rajentheran R, McLean N R, Kelly C G, Reed M F, Nolan A . Malignant transformation of oral lichen planus. Eur J Surg Oncol 1999; 25 : 520–523.
Ingafou M, Leao J C, Porter S R, Scully C . Oral lichen planus: a retrospective study of 690 British patients. Oral Dis 2006; 12 : 463–468.
Giuliani M, Troiano, G, Cordaro M et al. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis 2018; 1111/odi.12885 [Epub ahead of print].
Murti P R, Bhonsle R B, Pindborg J J, Daffary D K, Gupta P C, Mehta F S . Malignant transformation rate in oral submucous fibrosis over 17 period. Community Dent Oral Epidemiol 1985; 13 : 340–341.
Gupta P C, Mehta F S, Daftary D K et al. Incidence rates of oral cancer and natural history of oral precancerous lesions in a 10-year follow up study of Indian villagers. Community Dent Oral Epidemiol 1980; 8 : 287–333.
Ogden G R, Connor E, Chisholm D M . Dyskeratosis congenita: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol 1988; 65 : 586–591.
Williams D W, Bartie K L, Potts A J C, Wilson M J, Fardy M J, Lewis M A O . Strain persistence of invasive Candida albicans in chronic hyperplastic candidosis that underwent malignant change. Gerodontology 2001; 18 : 73–78.
Brocklehurst P, Pemberton M N, Macey R, Cotton C, Walsh T, Lewis M A O . Comparative accuracy of different members of the dental team in detecting malignant and non-malignant oral lesions. Br Dent J 2015; 218 : 525–529.
Warnakulasuriya S . Diagnostic adjuncts on oral cancer and precancer: an update for practitioners. Br Dent J 2017; 223 : 663–666.
Rashid A, Warnakulasuriya S . The use of light-based (optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: a systematic review. J Oral Pathol Med 2015; 44 : 307–328.
Warnakulasuriya S . Translational research in oral oncology – A bridge between basic science and clinical application. Transl Res Oral Oncol 2016; 1 : 1–2.
Macey R, Walsh T, Brocklehurst P et al. Diagnostic tests for oral cancer and potentially malignant disorders in patients with clinically evident lesions. Cochrane Database Sys Rev 2015; 5 : Cd010276. 10.1002/14651858.CD010276.pub2.
Lingen M W, Tampi M P, Urquhart O et al. Adjuncts for the evaluation of potentially malignant disorders in the oral cavity: Diagnostic test accuracy systematic review and meta-analysisa report of the American Dental Association. J Am Dent Assoc 2017; 148 : 797–813.e52.
Scheifele C, Schmidt-Westhausen A M, Dietrich T, Reichart P A . The sensitivity and specificity of the OralCDx technique: evaluation of 103 cases. Oral Oncol 2004; 40 : 824–828.
Radhika T, Jeddy N, Nithya S, Muthumeenakshi R M . Salivary biomarkers in oral squamous cell carcinoma – An insight. J Oral Biol Craniofac Res 2016; 6 : S51–S54. 10.1016/j.jobcr.2016.07.003.
Article PubMed PubMed Central Google Scholar
Bano S, David M P, Indira A P . Salivary biomarkers for oral squamous cell carcinoma: An overview. IJSS Case Rep Rev 2015; 1 : 39–45.
Spielmann N, Wong D T . Saliva: diagnostics and therapeutic perspectives. Oral Dis 2011; 17 : 345–354.
Cheng Y S, Rees T, Wright J . A review of research on salivary biomarkers for oral cancer detection. Clin Transl Med 2014; 3 : 3. 10.1186/2001-1326-3-3.
Miller C C, Hierons R J . Two audits of the diagnosis of oral cancer and the two-week rule following referrals from primary care practitioners in Newcastle. Prim Dent Care 2012; 19 : 63–68.
Singh P, Warnakulasuriya S . The two-week wait cancer initiative on oral cancer; the predictive value of urgent referrals to an oral medicine unit. Br Dent J 2006; 201 : 717–720.
Langton S, Siau D, Bankhead C . Two-week rule in head and neck cancer 2000–2014: asystematic review. Br J Oral Maxillofac Surg. 2016 : 54: 120–131.
National Institute for Health and Care Excellence. Suspected cancer: recognition and referral . 22 June 2015. Available at www.nice.org.uk/guidance/ng12 (accessed August 2018).
Healthcare Improvement Scotland. Scottish referral guidelines for suspected cancer . August 2014. Available at www.healthcareimprovementscotland.org/our_work/cancer_care_improvement/programme_resources/scottish_referral_guidelines.aspx (accessed August 2018).
Grimes D . Patel J, Avery C . New NICE referral guidance for oral cancer: does it risk delay in diagnosis? Br J Oral and Maxillofac Surg 2016; 55 : 404–406.
Yeung C A . Referrals to dentists by GPs could delay diagnosis of oral cancer. BMJ 2017; 356: i6784. Available at https://doi.org/10.1136/bmj.i6784 (accessed 19 October 2018).
Kalavrezos N, Scully C . Mouth cancer for clinicians Part 8: Referral. Dent Update 2016; 43 : 176–185.
Awojobi O, Newton J T, Scott S E . Pilot study to train dentists to communicate about oral cancer: the impact on dentist's self-confidence reported behaviour, confidence and beliefs. Br Dent J 2016; 220 : 71–76.
Download references
Author information
Authors and affiliations.
Professor of Oral Medicine, School of Dentistry, Cardiff University, Heath Park, Cardiff, CF14 4XY,
M. A. O. Lewis
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to M. A. O. Lewis .

Rights and permissions
Reprints and permissions
About this article
Cite this article.
Lewis, M. Mouth cancer: presentation, detection and referral in primary dental care. Br Dent J 225 , 833–840 (2018). https://doi.org/10.1038/sj.bdj.2018.931
Download citation
Accepted : 04 October 2018
Published : 09 November 2018
Issue Date : 09 November 2018
DOI : https://doi.org/10.1038/sj.bdj.2018.931
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Oral medicine considerations for the older patient.
- Conor O’Gorman
- Amanda Willis
British Dental Journal (2024)
Appropriateness of two-week wait head and neck cancer referrals to a district general hospital
- Hannah Hook
- Gabriele Baniulyte
- John Bowden
British Dental Journal (2023)
Slow to heal or slow to diagnose cancer?
- Charlotte A. Richards
- Alice Cameron
- Barry G. Main
British Dental Journal (2021)
An audit to analyse the two-week wait pathway at an oral cancer specialist district general hospital
- Ariyan S. Araghi
- Yasmin Harris
- Panayiotis Kyzas
British Dental Journal (2020)
The reconstructive oral cancer patient: what the general dental practitioner needs to know
British Dental Journal (2019)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gelband H, Jha P, Sankaranarayanan R, et al., editors. Cancer: Disease Control Priorities, Third Edition (Volume 3). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2015 Nov 1. doi: 10.1596/978-1-4648-0349-9_ch5

Cancer: Disease Control Priorities, Third Edition (Volume 3).
Chapter 5 oral cancer: prevention, early detection, and treatment.
Rengaswamy Sankaranarayanan , Kunnambath Ramadas , Hemantha Amarasinghe , Sujha Subramanian , and Newell Johnson .
Affiliations

- Introduction
Oral cancer is the 11th most common cancer in the world, accounting for an estimated 300,000 new cases and 145,000 deaths in 2012 and 702,000 prevalent cases over a period of five years (old and new cases) ( tables 5.1 and 5.2 ) ( Bray and others 2013 ; Ferlay and others 2013 ). For this chapter, oral cancers include cancers of the mucosal lip, tongue, gum, floor of the mouth, palate, and mouth, corresponding to the International Classification of Diseases, 10th revision [ICD-10], codes C00, C02, C03, C04, C05, and C06, respectively. Two-thirds of the global incidence of oral cancer occurs in low- and middle-income countries (LMICs); half of those cases are in South Asia. India alone accounts for one-fifth of all oral cancer cases and one-fourth of all oral cancer deaths ( Ferlay and others 2013 ).
Oral Cancer in Men (All Ages): Global Incidence, Mortality, and Prevalence, World Health Organization Geographic Classification, 2012.
Oral Cancer in Women (All Ages): Global Incidence, Mortality, and Prevalence, World Health Organization Geographic Classification, 2012.
Tobacco use, in any form, and excessive alcohol use are the major risk factors for oral cancer. With dietary deficiencies, these factors cause more than 90 percent of oral cancers. Preventing tobacco and alcohol use and increasing the consumption of fruits and vegetables can potentially prevent the vast majority of oral cancers ( Sankaranarayanan and others 2013 ). When primary prevention fails, early detection through screening and relatively inexpensive treatment can avert most deaths. However, oral cancer continues to be a major cancer in India, East Asia, Eastern Europe, and parts of South America ( Forman and others 2013 ), where organized prevention and early detection efforts are lacking. This chapter discusses the epidemiology, prevention, early detection, and treatment of oral cancers, as well as the cost-effectiveness of interventions.
- Oral Cancer: Incidence, Mortality, and Survival
Incidence and Mortality 1
Oral cancer incidence and mortality are high in India; Papua New Guinea; and Taiwan, China, where chewing of betel quids with tobacco or without tobacco or areca nut chewing is common, as well as in Eastern Europe, France, and parts of South America (Brazil and Uruguay), where tobacco smoking and alcohol consumption are high. The age-standardized incidence rates for men are, on average, twice as high as those for women ( tables 5.1 and 5.2 ). Incidence rates do not follow a particular pattern from low- to high-income countries (HICs), when countries are grouped into wealth strata ( figure 5.1 ). In selected countries where some reliable cancer registries exist, India is highest and Belarus is lowest, with incidence rates varying by more than five times in men and women. The estimated age-standardized incidence rates of oral cancer also vary among countries in different regions ( maps 5.1 and 5.2 ).
Age-Standardized Incidence and Mortality Rates of Oral Cancer, by World Bank Income Classification, 2012.
Age-Standardized Incidence Rates of Oral Cancer in Men, 2012.
Age-Standardized Incidence Rates of Oral Cancer in Women, 2012.
The buccal (cheek) mucosa is the most common site for oral cancer in South and Southeast Asia; in all other regions, the tongue is the most common site ( Forman and others 2013 ). Regional variations in incidence and the site of occurrence relate to the major causes, which are alcohol and smoking in Western countries, and betel quid and tobacco chewing in South and Southeast Asia ( Lambert and others 2011 ). Oral cancer mortality rates range between 1 and 15 per 100,000 persons in different regions; mortality rates exceed 10 per 100,000 in Eastern European countries, such as the Czech Republic, Hungary, and the Slovak Republic ( Ferlay and others 2013 ). Oral cancer mortality rates are influenced by oral cancer incidence, access to treatment, and variations in site distribution.
The observed trends in incidence and mortality among men and women are closely correlated with the patterns and trends in tobacco and alcohol use. An increasing trend in incidence has been reported in Karachi, Pakistan ( Bhurgri and others 2006 ), and in Taiwan, China ( Tseng 2013 ), caused by increases in tobacco and areca nut chewing and alcohol drinking. Oral cancer incidence and mortality rates have been steadily declining over the past two decades because of declining smoking prevalence and alcohol consumption in the United States ( Brown, Check, and Devesa 2011 ). However, a recent increase in cancers at the base of the tongue, possibly driven by the human papillomavirus (HPV), has been observed in white men in the United States ( Saba and others 2011 ).
Oral cancer incidence and mortality rates have been declining steadily in most European countries over the past two decades; until recently, rates had been increasing in some Central European countries, including Hungary and the Slovak Republic, reflecting changes in alcohol and tobacco consumption ( Bonifazi and others 2011 ). Oral cancer mortality has declined steadily in France since reaching a peak in the early 1990s, and the decline correlates with the reduction in per capita alcohol consumption. Incidence and mortality have been stable in the Nordic countries, the Russian Federation, and the United Kingdom. Mortality rates have been steadily declining in Australia and Hong Kong SAR, China, but increasing in Japan and the Republic of Korea ( Yako-Suketomo and Matsuda 2010 ).
In the United States, five-year survival improved by more than 11 percentage points between 1992 and 2006 ( Pulte and Brenner 2010 ) and is now approximately 65 percent ( Howlader and others 2010 ; Ries and others 2008 ). In Europe, it is approximately 50 percent ( Sant and others 2009 ). In India, five-year survival is less than 35 percent; in China, the Republic of Korea, Pakistan, Singapore, and Thailand, it ranges between 32 and 54 percent ( Sankaranarayanan and others 2010 ; Sankaranarayanan and Swaminathan 2011 ). Overall, the five-year survival for early, localized cancers exceeds 80 percent and falls to less than 20 percent when regional lymph nodes are involved.
- Oral Cancer: Risk Factors and Prevention
The major causes of oral cancer worldwide remain tobacco in its many different forms, heavy consumption of alcohol, and, increasingly, infection with certain types of HPV. Although the relative contribution of risk factors varies from population to population, oral cancer is predominantly a disease of poor people ( Johnson and others 2011 ). Prevention of this devastating disease can come from fundamental changes in socioeconomic status, as well as from actions to reduce the demand, production, marketing, and use of tobacco products and alcohol ( Johnson and others 2011 ). A healthy diet, good oral and sexual hygiene, and awareness of the signs and symptoms of disease are important. Success depends on political will, intersectoral action, and culturally sensitive public health messages disseminated through educational campaigns and mass media initiatives.
Smokeless and Smoking Tobacco Use
Smokeless tobacco in the form of betel quid, oral snuff, and betel quid substitutes (locally called guktha, nass, naswar, khaini, mawa, mishri, and gudakhu) increases the risk of oral precancerous lesions and oral cancer between 2-fold and 15-fold ( Gupta and others 2011 ; Gupta, Ariyawardana, and Johnson 2013 ; IARC 2004b , 2007 ; Javed and others 2010 ; Johnson and others 2011 ; Somatunga and others 2012 ). In most areas, betel quid consists of tobacco, areca nut, slaked lime, catechu, and several condiments, wrapped in a betel leaf. In recent years, small, attractive, and inexpensive sachets of betel quid substitutes containing a flavored and sweetened dry mixture of areca nut, catechu, and slaked lime with tobacco (gutkha) or without tobacco (pan masala), often claiming to be safer products, have become widely available and are increasingly used by young people, particularly in India. These products have been strongly implicated in oral submucous fibrosis (OSMF), which places individuals at high risk for malignancy.
More than 50 percent of oral cancers in India, Sudan, and the Republic of South Sudan, and about 4 percent of oral cancers in the United States, are attributable to smokeless tobacco products. Smokeless tobacco use among young people is increasing in South Asia, with the marketing of conveniently packaged products made from areca nut and tobacco; as a consequence, oral precancerous conditions in young adults have increased significantly ( Gupta and others 2011 ; Sinha and others 2011 ).
Consistent evidence from many studies indicates that tobacco smoking in any form increases the risk of oral cancer by twofold to tenfold in men and women ( IARC 2004a ). Risk increases substantially with duration and frequency of tobacco use; risk among former smokers is consistently lower than among current smokers, and there is a trend of decreasing risk with increasing number of years since quitting. Use of smokeless tobacco and alcohol in combination with tobacco smoking greatly increases the risk of oral cancer. The biological plausibility is provided by the identification of several carcinogens in tobacco, the most abundant and strongest being tobacco-specific N-nitrosamines, such as N-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone ( IARC 2007 ). These are formed by N-nitrosation of nicotine, the major alkaloid responsible for addiction to tobacco.
The fact that more than 80 percent of oral cancers can be attributed to tobacco and/or alcohol consumption justifies regular oral examinations targeting tobacco and alcohol users, as well as prevention efforts focusing on tobacco and alcohol control ( Radoi and others 2013 ). The World Health Organization Framework Convention on Tobacco Control, an evidence-based international treaty, aims to reduce the demand for tobacco globally by price, tax, and non-price measures. (See chapter 10 for a full discussion of tobacco control.)
Areca Nut Chewing
Areca nut or betel nut, because it is often wrapped in betel leaf, is now regarded as a type 1 carcinogen ( IARC 2004b , 2007 ). It is chewed raw, dried, or roasted, or as part of betel quid, by millions of people in Asia; its use is spreading across the Pacific, as well as in emigrant Asian communities worldwide. Cheap, prepackaged areca nut products, such as pan masala, are of recent concern, especially among youth. The inclusion of tobacco in the betel quid adds considerably to the carcinogenicity ( Amarasinghe and others 2010 ; Johnson and others 2011 ).
Alcohol Use
Epidemiological studies indicate that drinking alcoholic beverages increases the risk of oral cancer twofold to sixfold and is an independent risk factor ( IARC 2010 ), with risk increasing with quantity consumed. The risk varies by population and individual and subsite within the oral cavity ( Radoi and others 2013 ). The combined use of alcohol and tobacco has a multiplicative effect on oral cancer risk. The various pathways by which alcohol may exert carcinogenic influence include topical exposure leading to a direct effect on cell membranes, altered cell permeability, variation in enzymes that metabolize alcohol, and/or systemic effects, such as nutritional deficiency, immunological deficiency, and disturbed liver function. A recent review failed to identify an association between the use of mouthwash containing alcohol and oral cancer risk, or any significant trend in risk with increasing daily use of mouthwash ( Gandini and others 2012 ).
Poor Nutrition
High consumption of fruits and vegetables is associated with a reduction of 40–50 percent in the risk of oral cancer ( Lucenteforte and others 2009 ; Pavia and others 2006 ; World Cancer Research Fund/American Institute for Cancer Research 2007 ). In HICs, selected aspects of diet—such as lack of vegetables and fruits—may account for 15–20 percent of oral cancers; this proportion is likely to be higher in LMICs. Chemoprevention studies have not established a preventive effect of retinoid and carotenoid dietary supplements ( Chainani-Wu, Epstein, and Touger-Decker 2011 ; Wrangle and Khuri 2007 ).
Other Risk Factors
Genetic factors.
Most carcinogens are metabolized through the cytochrome p450 system in the liver. If this system is defective by virtue of inheriting a particular form of the gene (a polymorphism), the risk of many cancers is enhanced. This risk is particularly important with oral and other head and neck cancers, although the relative risks are modest at 1.5 or lower (that is, less than a doubling of risk) ( Lu, Yu, and Du 2011 ).
Polymorphisms in alcohol-metabolizing enzymes also contribute to the risk. Individuals with the fast-metabolizing version (allele) of alcohol dehydrogenase (ADH3[1-1]) have a greater risk of developing oral cancer in the presence of alcoholic beverage consumption than those with the slow-metabolizing forms; this higher risk re-enforces the role of acetaldehyde as the carcinogen involved ( Harty and others 1997 ).
Mate Drinking
Mate, a leaf infusion that is commonly drunk many times a day in parts of South America—usually very hot—appears to enhance the risk of oral cancer by a small amount ( Deneo-Pellegrini and others 2012 ).
Recent evidence suggests that HPV infection may be an independent risk factor for cancer of the base of the tongue, tonsils, and elsewhere in the oropharynx. HPV may modulate the process of carcinogenesis in some tobacco- and alcohol-induced oral and oropharyngeal cancers, and it may act as the primary oncogenic agent for inducing carcinogenesis among nonsmokers ( Johnson and others 2011 ; Prabhu and Wilson 2013 ). Growing evidence suggests that such oropharyngeal infections can be sexually transmitted ( Heck and others 2010 ).
Chronic Trauma
It now seems clear that chronic trauma, from sharp teeth, restorations, or dentures, contributes to oral cancer risk, although this higher risk commonly occurs only in the presence of the other local risk factors ( Piemonte, Lazos, and Brunotto 2010 ).
- Oral Cancer: Natural History
Oral cancer has a long preclinical phase that consists of well-documented precancerous lesions. The precancerous lesions include homogeneous leukoplakia, nonhomogeneous leukoplakia, verrucous leukoplakia, erythroplakia, OSMF, lichen planus, and chronic traumatic ulcers. The estimated annual frequency of malignant transformation of oral precancerous lesions ranges from 0.13 percent to 2.2 percent ( Amagasa, Yamashiro, and Uzawa 2011 ; Napier and Speight 2008 ).
Very early preclinical invasive cancers (early-stage cancers without symptoms) present as painless small ulcers, nodular lesions, or growths. These changes can be easily seen and are clinically detectable through careful visual inspection and palpation of the oral mucosa. Early, localized oral cancers—less than four centimeters—that have not spread to the regional lymph nodes can be effectively treated and cured with surgery or radiotherapy alone, with no functional or cosmetic defects, resulting in five-year survival rates exceeding 80 percent.
Leukoplakia is a white plaque that may be categorized clinically as homogeneous or nonhomogeneous . Homogeneous lesions are thin, flat, uniform, smooth, and white. Nonhomogeneous lesions may have a white and red appearance or tiny, white, pinhead-size raised nodules on a reddish background or a proliferative, warty appearance. Erythroplakia presents as a red patch with smooth or granular surface that cannot be characterized clinically or pathologically as any other definable disease ( Warnakulasuriya, Johnson, and Van Der Waal 2007 ). Erythroplakia has a higher probability than leukoplakia to harbor occult invasive cancer and to undergo malignant transformation.
Oral lichen planus may present as interlacing white lines (known as Wickham’s striae ) with a reddish border, or as a mix of reddish and ulcerated areas.
OSMF, mostly restricted to people of Indian subcontinent origin and in certain Pacific islands such as Mariana Islands, presents with a burning sensation, blanching of the oral mucosa, and intolerance to spicy food. Stiffening and atrophy of the oral and pharyngeal mucosa occurs as the disease progresses, leading to reduced mouth opening and difficulty in swallowing and speaking.
Palatal lesions are seen in populations who smoke with the lighted end of the tobacco product inside the mouth, known as reverse smoking , resulting in white or mixed reddish-white lesions of the palate.
A higher risk of malignant transformation may be associated with the following factors: female gender, lesions of long duration, large precancerous lesions, precancerous lesions in nonusers of tobacco, tongue and floor of mouth lesions, nonhomogeneous lesions, and lesions showing epithelial dysplasia and aneuploidy ( Hsue and others 2007 ; Napier and Speight 2008 ). However, it is impossible to predict with certainty which precancerous lesion will become malignant during follow-up in patients. The malignant transformation of precancerous lesions can be prevented by interventions, such as avoiding exposure to tobacco use and alcohol drinking, and in selected instances, by excision of the lesions.
- Oral Cancer Screening: Accuracy, Efficacy, and Potential Harms
Although an affordable, acceptable, easy to use, accurate, and effective screening test for oral cancer is available in high-risk countries, a decision to introduce population-based screening should take into account the level of health service development and available resources to meet the increased treatment demand that screening generates. The target population for oral cancer screening consists of those age 30 years and older who use tobacco and/or alcohol.
Visual screening of the oral cavity has been widely evaluated for its feasibility, safety, acceptability, accuracy to detect oral precancerous lesions and cancer, and efficacy and cost-effectiveness in reducing oral cancer mortality ( Johnson and others 2011 ; Sankaranarayanan and others 2005 ; Sankaranarayanan and others 2013 ). Visual screening involves systematic visual and physical examination of the intraoral mucosa under bright light for signs of oral potentially malignant disorders (OPMDs), as well as early oral cancer, followed by careful inspection and digital palpation of the neck for any enlarged lymph nodes. It is a provider-dependent, subjective test; accordingly, its performance in detecting lesions varies among providers. Comprehensive knowledge of the oral anatomy, the natural history of oral carcinogenesis, and the clinico-pathological features of the OPMDs and preclinical cancers are important prerequisites for efficient providers of oral visual screening.
The potential harms of oral visual screening may include additional diagnostic investigations, such as incisional or excisional biopsy; anxiety associated with false-positive screening tests; detection and treatment of biologically insignificant conditions that may have no impact on oral cancer incidence; and false reassurance from false-negative tests.
Visual Screening by Health Care Personnel
A variety of health care personnel—including dentists, general practitioners, oncologists, surgeons, nurses, and auxiliary health workers—may provide oral visual screening after training ( Ramadas and others 2008 ). Sensitivity ranges from 40 percent to 93 percent, and specificity ranges from 50 percent to 99 percent for detecting precancerous lesions and early asymptomatic oral cancers ( Downer and others 2004 ; Mathew and others 1997 ; Mehta and others 1986 ; Warnakulasuriya and others 1984 ; Warnakulasuriya and Nanayakkara 1991 ).
A significant reduction of 34 percent in oral cancer mortality among a high-risk group of tobacco or alcohol users following three rounds of oral visual screening has been demonstrated in a cluster-randomized controlled trial in India ( Sankaranarayanan and others 2005 ; Sankaranarayanan and others 2013 ). A 15-year follow-up found sustained reduction in oral cancer mortality, with larger reductions in those adhering to repeated screening rounds; there was a 38 percent reduction in oral cancer incidence (95 percent confidence interval [CI] 8–59 percent), and an 81 percent reduction in oral cancer mortality (95 percent CI 69–89 percent) in tobacco and/or alcohol users who were screened four times ( Sankaranarayanan and others 2013 ). The studies ( Sankaranarayanan and others 2005 ; Sankaranarayanan and others 2013 ) were the basis for the conclusions of the recent Cochrane Collaboration Review ( Brocklehurst and others 2013 ) and an American Dental Association (ADA) expert panel review on population-based oral cancer screening ( Rethman and others 2010 ). The ADA review recommended that clinicians look for signs of precancerous lesions or early-stage cancers while performing routine visual and tactile screening in all subjects, particularly in those who use tobacco or alcohol or both; the panel also concluded that the life-saving benefits for subjects with treatable lesions were more important than the potential harms incurred by those with benign or nonprogressive lesions ( Rethman and others 2010 ). The Cochrane Review ( Walsh and others 2013 ) concluded that evidence suggests that a visual examination as part of a population-based screening program reduces the mortality rate of oral cancer in high-risk individuals; in addition, it could result in diagnoses of oral cancer at an earlier stage of disease and improvement in survival rates across the population as a whole ( Brocklehurst and others 2013 ).
The U.S. Preventive Services Task Force released a draft Recommendation Statement, which stated that for adults age 18 years or older seen in primary care settings, the current evidence is insufficient to assess the balance of benefits and harms of screening for oral cancer in asymptomatic adults. However, this statement overlooks the benefits of early detection of oral cancers among users of tobacco or alcohol or both, as well as other benign conditions whose early detection may improve oral health. Discouraging oral visual examination in primary care is clearly not in the interests of oral cancer control and improving oral health ( Edwards 2013 ).
Self-Examination and Other Screening Methods
Although mouth self-examination using a mirror has been evaluated as a screening test in some studies ( Elango and others 2011 ; Mathew and others 1995 ; Scott and others 2010 ), whether it could lead to reductions in oral cancer mortality is not known. There is insufficient evidence to recommend the routine use of other oral screening tests, such as toluidine blue staining, chemiluminescence, tissue fluorescence imaging, tissue fluorescent spectroscopy, and salivary analysis and cytology for primary screening of oral cancer ( Johnson and others 2011 ; Patton, Epstein, and Kerr 2008 ; Richards 2010 ; Su and others 2010 ).
Despite the high risk of oral cancer in the Indian subcontinent, no national or regional screening programs exist in the region. The only large-scale, ongoing, national oral cancer screening programs are in Cuba and Taiwan, China.
- The Cuban program has been in existence since 1984. An evaluation conducted in 1994 indicated that 12–26 percent of the target population has been screened annually, but less than 30 percent of screen-positive individuals complied with referrals ( Fernandez and others 1995 ). The program was reorganized in 1996, with the target age raised from 15 years to 35 years, screening intervals increased from one to three years, and the referral system revamped. No further formal evaluation has been conducted, but there has been no reduction in oral cancer incidence or mortality rates in Cuba over the past three decades. The outcomes from the Cuban program emphasize that screening programs without efficient organization and resources are not an effective use of limited resources.
- Oral cancer screening was initiated in Taiwan, China, in 2004, targeting those age 18 years and older who were smokers or betel nut chewers; the target population for oral cancer screening was revised in 2010 to cover smokers or chewers age 30 years and older. The screening program has led to almost half of the oral cancers diagnosed in stages I and II, with a declining trend in oral cancer mortality rates.
- Oral Cancer: Early Clinical Diagnosis and Staging
Primary care dental and general practitioners should play a major role in referring patients to cancer treatment facilities for early diagnosis and treatment. Improving the skills of these primary care doctors is essential to improving prospects for early diagnosis, particularly among patients who use tobacco or alcohol in any form. Routine biopsy in those clinically presenting with features of precancerous lesions may lead to early diagnosis of underlying invasive oral cancer. In addition to history, physical examination, and biopsy, a simultaneous assessment of the upper aerodigestive tract is necessary because patients with oral cancer have a high risk of cancers developing in other head and neck sites and in the lungs.
Once a diagnosis of oral cancer is confirmed, staging assessment is completed and treatment is planned. The Union for International Cancer Control Tumor, Nodes, Metastasis (TNM) staging system is widely used for staging oral cancer ( Patel and Shah 2005 ; Sobin and Wittekind 2002 ) ( table 5.3 ): T indicates the size and extent of spread of the primary tumor, N indicates the extent spread to the regional lymph nodes in the neck, and M indicates the spread to distant organs. The TNM categorization is further grouped into stages 0 through IV, which denote increasing severity of disease and decreasing survival.
Clinical Staging of Oral Cancer, Treatment Modalities, and Prognosis, by Clinical Stage.
Oral cancer staging involves assessing the clinical extent of disease through physical examination, biopsies, and imaging investigations, including X-rays of the mandible, maxillary sinuses, and chest; computerized tomography (CT) scans; magnetic resonance imaging (MRI); and positron emission tomography (PET) imaging, depending on what resources are available. Advanced imaging techniques such as CT, MRI, and PET may be useful in more accurately evaluating local spread, such as invasion of muscles, bone, and cartilage, and lymph node metastases, as well as in planning treatment, but these investigations are seldom feasible in LMICs.
- Oral Cancer: Management
Oral cancer is predominantly a loco-regional disease that tends to infiltrate adjacent bone and soft tissues and spreads to the regional lymph nodes in the neck. Distant metastasis is uncommon at the time of diagnosis. A thorough inspection and palpation of the oral cavity and examination of the neck is mandatory. CT and MRI imaging are widely used to assess the extent of involvement of adjacent structures, such as bones and soft tissues. Surgery and radiotherapy are the main treatment modalities. Given the skills, expertise, and infrastructure required for staging and treatment with minimal physical, functional, and cosmetic morbidity, oral cancer treatment is usually provided in specialized cancer hospitals, such as comprehensive cancer centers, or in hospitals at the highest level of health services, third-level centers.
Treatment of Early-Stage Oral Cancer (Stages I and II)
Surgery and radiotherapy are widely used for the treatment of early oral cancer, either as single modalities or in combination. The choice of modality depends on the location of the tumor, cosmetic and functional outcomes, age of the patient, associated illnesses, patient’s preference, and the availability of expertise.
Most early-stage oral cancers can be locally excised or treated with radiotherapy, with no or minimal functional and physical morbidity. Elective neck dissection to remove lymph nodes may be considered in selected cases, such as patients with stage I tongue cancer and stage II cancers at other oral sites, who may be at high risk of microscopic but not clinically evident involvement of the neck nodes (N0) (El-Naaj and others 2011; Hicks, Jr., and others 1997 ; Vijayakumar and others 2011 ; Woolgar 2006 ; Zwetyenga and others 2003 ).
External beam radiotherapy and brachytherapy—using radioactive sources implanted in the tumor—either alone or in combination, is an alternative to surgery for early-stage oral cancers. Excellent outcomes have been demonstrated following brachytherapy alone or in combination with external beam radiotherapy for small tumors ( Fujita and others 1999 ; Marsiglia and others 2002 ; Wendt and others 1990 ). Deep infiltrative cancers have a high propensity to spread to regional lymph nodes; therefore, brachytherapy alone, which does not treat regional nodes adequately, is not recommended. Newer techniques, such as three-dimensional conformal radiotherapy and intensity modulated radiotherapy, can minimize the side effects of radiotherapy by delivering the radiation dose to the tumor more precisely and accurately while avoiding healthy surrounding tissues. However, these treatments require advanced equipment and are more expensive than conventional radiotherapy.
Treatment of Locally Advanced Tumors of the Oral Cavity (Stages III and IV)
Locally advanced tumors are aggressive, and locoregional treatment failure rates are high. A combined modality approach integrating surgery, radiotherapy with or without chemotherapy, and planned and executed by a multidisciplinary team is always preferred. Appropriate importance should be given to factors such as functional and cosmetic outcomes and the available expertise. Surgery followed by postoperative radiotherapy is the preferred modality for patients with deep infiltrative tumors and those with bone infiltration ( Lundahl and others 1998 ). Postoperative concurrent chemo-radiation has been found to be superior to radiotherapy alone in those with surgical margins showing cancerous changes indicating incomplete excision of the tumor ( Bernier and others 2004 ; Cooper and others 2004 ). The use of chemotherapy prior to surgery may eliminate the need to remove the mandible—a major benefit—although it does not confer a survival benefit ( Licitra and others 2003 ).
Primary radiotherapy, with or without chemotherapy, is a reasonable option for locally advanced tumors without bone involvement, especially for patients who have inoperable disease, who are medically unfit for surgery, or who are likely to have unacceptable functional and cosmetic outcomes with surgery. Incorporating chemotherapy with surgery or radiotherapy is useful in younger patients who are in good general condition, increasing survival by about 5 percentage points at five years ( Blanchard and others 2011 ).
Side Effects of Radiotherapy
Side effects may occur during or immediately following radiotherapy—acute reactions—or months to years after treatment. Acute reactions are self-limiting and generally resolve within two to three weeks. These reactions are caused by the inflammation of tissues within the radiotherapy treatment field. Alteration of taste, pain, difficulty in eating, mucosal ulceration of the oral cavity, bacterial and fungal infections, increased thickness of saliva, discoloration and peeling of the overlying skin, loss of hair within the field of treatment, and edema of the skin are the major side effects. Maintenance of good oral hygiene, frequent cleaning of the oral cavity with soda-saline solution, analgesics, and control of infection are recommended for conservative management of these side effects. Good hydration, a high-calorie diet, and avoidance of spicy and hot food are recommended.
Late effects of radiation are related to dose per fraction, total dose, and the type and volume of the tissue irradiated. Late effects include loss of hair within the irradiated area, dry mouth (xerostomia), thickening of the skin, dental caries, and, rarely, necrosis of the mandible or maxillary bone.
Complications of Surgery
The common complications of oral surgery are infection, collection of blood (hematoma), skin necrosis, flap failure, and wound breakdown. Resorption of bone, osteomyelitis, and salivary fistula can also occur. Complications are more frequent when neck dissection is part of the surgery. Fatal hemorrhage can occur if the carotid artery is exposed in the wound. Resection of the structures can interfere with cosmetic appearance and functions such as speech, swallowing, and airway. These complications can be minimized through reconstructive surgery and by good prosthetic rehabilitation.
Posttreatment Follow-Up
Patients with oral cancer are at risk for developing loco-regional recurrences and second malignancies. After completion of the treatment, patients should be followed up at regular intervals to detect any signs of recurrence. Patients should be encouraged to give up tobacco and alcohol and know the signs and symptoms of recurrence.
Lymph node involvement and tumor size are the most important prognostic factors. Data for the United States for 1975–2007 report a five-year survival for all stages of oral cancer of 60.9 percent, 82.5 percent for early-stage disease, and 54.7 percent for locally advanced oral cavity cancer ( Ries and others 2008 ). The reported five-year overall survival rates for oral cancer for all stages combined from populations in LMICs such as China, Cuba, India, Pakistan, and Thailand ranged from 26 to 45 percent; for stages I and II, the survival rates ranged from 36 to 83 percent. The inferior survival rates in LMICs versus HICs reflect disparities in the availability, accessibility, and affordability of diagnostic and treatment services ( Sankaranarayanan and others 2010 ; Sankaranarayanan and Swaminathan 2011 ).
- Economics of Preventing and Screening for Oral Cancers in LMICs
Cost-Effectiveness Assessments
Only a few cost-effectiveness studies of oral cancer screening focus on LMICs; therefore, we include a broader range of studies, including some from HICs. Although these studies may not be directly relevant to resource-limited settings, they provide valuable insights into the potential cost-effectiveness of interventions.
Primary Prevention
Interventions targeted at reducing or eliminating tobacco and alcohol use should be considered for implementation when shown to be cost-effective. All the interventions presented are cost-effective, even for LMICs. In the case of tobacco cessation, increasing the price of tobacco products is the most cost-effective approach, with incremental cost-effectiveness ratios ranging from US$4 to US$34 per disability-adjusted life year. Alcohol control interventions tend to have higher cost-effectiveness ratios; advertising bans and reduced access range from US$367 to US$1,307; combination strategies (including price increases, reduced access, and advertisement bans) range from US$601 to US$1,704. (Interventions to decrease tobacco use are covered in more detail in chapter 10 .)
Table 5.4 summarizes findings from the relevant cost-effectiveness studies. Among the four studies of the cost-effectiveness of oral cancer screening, three—all set in HICs—used decision analytic modeling; the other, the only one from a resource-constrained environment, used data from a randomized clinical trial in India. Only the Indian study ( Subramanian and others 2009 ) directly reflects the costs and effectiveness likely to be experienced in LMICs. In general, screening was at ages 35 or 40 years and older; three of the four studies included both high-risk and average-risk individuals. All of the studies presented incremental cost-effectiveness, compared with the scenario of no screening. A variety of interventions was assessed, using invitation and opportunistic screening; visual inspection was performed by specialists (oral cancer surgeons), dentists, or trained health care workers.
Oral Cancer Screening Cost-Effectiveness Studies.
The results indicate that screening is cost-effective even in LMICs. The study from India provides evidence that oral cancer screening by visual inspection costs less than US$6 per person in a screening program; this has an incremental cost-effectiveness ratio of US$835 per life year saved. The most cost-effective and affordable option in the limited-resource setting is to offer oral cancer screening to high-risk individuals, for example, tobacco and alcohol users. The incremental cost-effectiveness ratio for screening high-risk individuals in southern India is US$156 per life year saved. There is wide variation in the incremental cost-effectiveness reported across the studies, probably because of factors such as the underlying prevalence of disease and the local cost of cancer treatment. The cost of care related to screening, diagnosis, and treatment can differ substantially, even among countries classified as LMICs. Accordingly, it is essential to systematically assess costs at the country or even local level to analyze the cost-effectiveness and resources required to implement oral cancer screening.
Future Research Needs
Primary prevention, especially smoking cessation, and secondary prevention, focused on high-risk individuals, are likely to be cost-effective and affordable in LMICs. Additional studies are required to assess the cost-effectiveness and budget implications of visual screening for oral cancers in LMICs. These studies should focus on the screening delivery structure to identify the most cost-effective approach to provide oral cancer screening to high-risk individuals.
When cancer screening policies are implemented, the success of the program will depend on participation by the target population. Even when screening and follow-up care are free of charge, patients may not be able to afford to lose a day’s wages to attend screening clinics or travel to health centers to receive follow-up diagnostic testing or treatments. The indirect costs borne by the patients may be particularly challenging among those in the lower socioeconomic strata. These are the very individuals likely to be at higher risk for developing oral cancers; it is, therefore, vital that identifying approaches to encourage and sustain participation among this potentially hard-to-reach, high-risk population be given high priority.
A multifaceted approach that integrates health education, tobacco and alcohol control, early detection, and early treatment is needed to reduce the burden of this eminently preventable cancer. How to accomplish this is known; astonishingly, it has not been applied in most countries, and not at all in the high-burden countries. Improving awareness among the general public and primary care practitioners, investing in health services to provide screening and early diagnosis services for tobacco and alcohol users, and providing adequate treatment for those diagnosed with invasive cancer are critically important oral cancer control measures. Imaging, histopathology, cancer surgery and radiotherapy infrastructure and services, trained professionals, and the availability of chemotherapeutic agents are inadequate in many LMICs, seriously compromising early detection and optimum treatment. As this chapter has demonstrated, however, these interventions are affordable and cost-effective.
- Low-income countries = US$1,045 or less in 2013
- Lower-middle-income = US$1,046–US$4,125
- Upper-middle-income = US$4,126–US$12,745
- High-income countries = US$12,746 or more
Maps and figures in this chapter are based on incidence and mortality estimates for ages 0–69, consistent with reporting in all DCP3 volumes. Global cancer statistics are estimates for the year 2012 and have been provided by the International Agency for Research on Cancer from its GLOBOCAN 2012 database. Observable population-based data were derived from Cancer Incidence in Five Continents, 10th edition and for trends over time from CI5 Plus ( http://ci5 .iarc.fr/CI5plus/Default.aspx ). The discussion of burden (including risk factors), however, includes all ages unless otherwise noted. Interventions also apply to all age groups, except where age ranges or cutoffs are specified.
- Amagasa T, Yamashiro M, Uzawa N. 2011. “Oral Premalignant Lesions: From a Clinical Perspective.” International Journal of Clinical Oncology 16 (1): 5–14. [ PubMed : 21225307 ]
- Amarasinghe H K, Johnson N W, Lalloo R, Kumaraarachchi M, Warnakulasuriya S. 2010. “Derivation and Validation of a Risk-Factor Model for Detection of Oral Potentially Malignant Disorders in Populations with High Prevalence.” British Journal of Cancer 103 (3): 303–09. [ PMC free article : PMC2920027 ] [ PubMed : 20628386 ]
- Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre J L., and others. 2004. “Postoperative Irradiation with or Without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer.” New England Journal of Medicine 350 (19): 1945–52. [ PubMed : 15128894 ]
- Bhurgri Y, Bhurgri A, Usman A, Pervez S, Kayani N. and others. 2006. “Epidemiological Review of Head and Neck Cancers in Karachi.” Asian Pacific Journal of Cancer Prevention 7 (2): 195–200. [ PubMed : 16839210 ]
- Blanchard P, Baujat B, Holostenco V, Bourredjem A, Baey C. and others. 2011. “Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): A Comprehensive Analysis by Tumour Site.” Radiotherapy and Oncology 100 (1): 33–40. [ PubMed : 21684027 ]
- Bonifazi M, Malvezzi M, Bertuccio P, Edefonti V, Garavello W. and others. 2011. “Age-Period-Cohort Analysis of Oral Cancer Mortality in Europe: The End of an Epidemic?” Oral Oncology 47 (5): 400–07. [ PubMed : 21402489 ]
- Bray F, Ren J S, Masuyer E, Ferlay J. 2013. “Estimates of Global Cancer Prevalence for 27 Sites in the Adult Population in 2008.” International Journal of Cancer 132 (5): 1133–45. [ PubMed : 22752881 ]
- Brocklehurst P, Kujan O, O’Malley L A, Ogden G, Shepherd S, Glenny A M. 2013. “Screening Programmes for the Early Detection and Prevention of Oral Cancer.” Cochrane Database of Systematic Reviews 11: CD004150. [ PMC free article : PMC8078625 ] [ PubMed : 24254989 ]
- Brown L M, Check D P, Devesa S S. 2011. “Oropharyngeal Cancer Incidence Trends: Diminishing Racial Disparities.” Cancer Causes and Control 22 (5): 753–63. [ PubMed : 21380619 ]
- Chainani-Wu N, Epstein J, Touger-Decker R. 2011. “Diet and Prevention of Oral Cancer: Strategies for Clinical Practice.” Journal of the American Dental Association 142 (2): 166–69. [ PubMed : 21282682 ]
- Cooper J S, Pajak T F, Forastiere A A, Jacobs J, Campbell B H., and others. 2004. “Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck.” New England Journal of Medicine 350 (19): 1937–44. [ PubMed : 15128893 ]
- Dedhia R C, Smith K J, Johnson J T, Roberts M. 2011. “The Cost-Effectiveness of Community-Based Screening for Oral Cancer in High-Risk Males in the United States: A Markov Decision Analysis Approach.” Laryngoscope 121 (5): 952–60. [ PMC free article : PMC3082601 ] [ PubMed : 21384383 ]
- Deneo-Pellegrini H, Stefani E De, Boffetta P, Ronco A L, Acosta G. and others. 2012. “Mate Consumption and Risk of Oral Cancer: Case-Control Study in Uruguay.” Head & Neck 35 (8): 1091–95. [ PubMed : 22915329 ]
- Downer M C, Moles D R, Palmer S, Speight P M. 2004. “A Systematic Review of Test Performance in Screening for Oral Cancer and Precancer.” Oral Oncology 40 (3): 264–73. [ PubMed : 14747057 ]
- Edwards C P. 2013. “Oral Cancer Screening for Asymptomatic Adults: Do the United States Preventive Services Task Force Draft Guidelines Miss the Proverbial Forest for the Trees?” Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 116 (2): 131–34. [ PubMed : 23849372 ]
- Elango K J, Anandkrishnan N, Suresh A, Iyer S K, Ramaiyer S K., and others. 2011. “Mouth Self-Examination to Improve Oral Cancer Awareness and Early Detection in a High-Risk Population.” Oral Oncology 47 (7): 620–24. [ PubMed : 21646040 ]
- El-Naaj I A, Leiser Y, Shveis M, Sabo E, Peled M. 2011. “Incidence of Oral Cancer Occult Metastasis and Survival of T1-T2N0 Oral Cancer Patients.” Journal of Oral & Maxillofacial Surgery 69 (10): 2674–79. [ PubMed : 21571415 ]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S. and others. 2013. GLOBOCAN 2012 V1.0, Cancer Incidence and Mortality Worldwide: IARC Cancerbase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. http://globocan .iarc.fr .
- Fernandez G L, Sankaranarayanan R, Anta J J Lence, Rodriguez S A, Parkin D M. 1995. “An Evaluation of the Oral Cancer Control Program in Cuba.” Epidemiology 6 (4): 428–31. [ PubMed : 7548355 ]
- Forman D, Bray F, Brewster D H, Gombe C Mbalawa, Kohler B. and others. 2013. Cancer Incidence in Five Continents. Vol. 10 (Electronic Version). Lyon, France: International Agency for Cancer. http://ci5 .iarc.fr .
- Fujita M, Hirokawa Y, Kashiwado K, Akagi Y, Kashimoto K. and others. 1999. “Interstitial Brachytherapy for Stage I and II Squamous Cell Carcinoma of the Oral Tongue: Factors Influencing Local Control and Soft Tissue Complications.” International Journal of Radiation Oncology Biology Physics 44 (4): 767–75. [ PubMed : 10386633 ]
- Gandini S, Negri E, Boffetta P, La V C, Boyle P. 2012. “Mouthwash and Oral Cancer Risk Quantitative Meta-Analysis of Epidemiologic Studies.” Annals of Agricultural and Environmental Medicine 19 (2): 173–80. [ PubMed : 22742785 ]
- Gupta B, Ariyawardana A, Johnson N W. 2013. “Oral Cancer in India Continues in Epidemic Proportions: Evidence Base and Policy Initiatives.” International Dental Journal 63 (1): 12–25. [ PMC free article : PMC9374955 ] [ PubMed : 23410017 ]
- Gupta P C, Ray C S, Sinha D N, Singh P K. 2011. “Smokeless Tobacco: A Major Public Health Problem in the SEA Region: A Review.” Indian Journal of Public Health 55 (3): 199–209. [ PubMed : 22089688 ]
- Harty L C, Caporaso N E, Hayes R B, Winn D M, Bravo-Otero E. and others. 1997. “Alcohol Dehydrogenase 3 Genotype and Risk of Oral Cavity and Pharyngeal Cancers.” Journal of the National Cancer Institute 89 (22): 1698–705. [ PubMed : 9390539 ]
- Heck J E, Berthiller J, Vaccarella S, Winn D M, Smith E M., and others. 2010. “Sexual Behaviours and the Risk of Head and Neck Cancers: A Pooled Analysis in the International Head and Neck Cancer Epidemiology (INHANCE) Consortium.” International Journal of Epidemiology 39 (1): 166–81. [ PMC free article : PMC2817092 ] [ PubMed : 20022926 ]
- Hicks W L Jr, Loree T R, Garcia R I, Maamoun S, Marshall D. and others. 1997. “Squamous Cell Carcinoma of the Floor of Mouth: A 20-Year Review.” Head & Neck 19 (5): 400–05. [ PubMed : 9243267 ]
- Hirsch J M, Wallstrom M, Carlsson A P, Sand L. 2012. “Oral Cancer in Swedish Snuff Dippers.” Anticancer Research 32 (8): 3327–30. [ PubMed : 22843910 ]
- Howlader N, Ries L A, Mariotto A B, Reichman M E, Ruhl J. and others. 2010. “Improved Estimates of Cancer-Specific Survival Rates from Population-Based Data.” Journal of the National Cancer Institute 102 (20): 1584–98. [ PMC free article : PMC2957430 ] [ PubMed : 20937991 ]
- Hsue S S, Wang W C, Chen C H, Lin C C, Chen Y K, Lin L M. 2007. “Malignant Transformation in 1458 Patients with Potentially Malignant Oral Mucosal Disorders: A Follow-Up Study Based in a Taiwanese Hospital.” Journal of Oral Pathology & Medicine 36 (1): 25–29. [ PubMed : 17181738 ]
- IARC (International Agency for Research on Cancer). 2004a. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 83: Tobacco Smoke and Involuntary Smoking. Lyon, France: IARC. [ PMC free article : PMC4781536 ] [ PubMed : 15285078 ]
- IARC (International Agency for Research on Cancer). 2004b. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 85: Betel-Quid and Areca-Nut Derived Nitrosamines. Lyon, France: IARC. [ PMC free article : PMC4781453 ] [ PubMed : 15635762 ]
- IARC (International Agency for Research on Cancer) 2007. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 89: Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines. Lyon, France: IARC. [ PMC free article : PMC4781254 ] [ PubMed : 18335640 ]
- IARC (International Agency for Research on Cancer) 2010. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 96: Alcohol Consumption and Ethyl Carbamate. Lyon, France: IARC. [ PMC free article : PMC4781168 ] [ PubMed : 21735939 ]
- Javed F, Chotai M, Mehmood A, Almas K. 2010. “Oral Mucosal Disorders Associated with Habitual Gutka Usage: A Review.” Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 109 (6): 857–64. [ PubMed : 20382045 ]
- Johnson N W, Warnakulasuriya S, Gupta P C, Dimba E, Chindia M. and others. 2011. “Global Oral Health Inequalities in Incidence and Outcomes for Oral Cancer: Causes and Solutions.” Advances in Dental Research 23 (2): 237–46. [ PubMed : 21490236 ]
- Lambert R, Sauvaget C, Camargo C M De, Sankaranarayanan R. 2011. “Epidemiology of Cancer from the Oral Cavity and Oropharynx.” European Journal of Gastroenterology & Hepatology 23 (8): 633–41. [ PubMed : 21654320 ]
- Licitra L, Grandi C, Guzzo M, Mariani L, Lo V S., and others. 2003. “Primary Chemotherapy in Resectable Oral Cavity Squamous Cell Cancer: A Randomized Controlled Trial.” Journal of Clinical Oncology 21 (2): 327–33. [ PubMed : 12525526 ]
- Lu D, Yu X, Du Y. 2011. “Meta-Analyses of the Effect of Cytochrome P450 2E1 Gene Polymorphism on the Risk of Head and Neck Cancer.” Molecular Biology Reports 38 (4): 2409–16. [ PubMed : 21076874 ]
- Lucenteforte E, Garavello W, Bosetti C, La V C. 2009. ”Dietary Factors and Oral and Pharyngeal Cancer Risk.” Oral Oncology 45 (6): 461–67. [ PubMed : 18990606 ]
- Lundahl R E, Foote R L, Bonner J A, Suman V J, Lewis J E., and others. 1998. “Combined Neck Dissection and Postoperative Radiation Therapy in the Management of the High-Risk Neck: A Matched-Pair Analysis.” International Journal of Radiation Oncology Biology Physics 40 (3): 529–34. [ PubMed : 9486600 ]
- Marsiglia H, Haie-Meder C, Sasso G, Mamelle G, Gerbaulet A. 2002. “Brachytherapy for T1-T2 Floor-of-the-Mouth Cancers: The Gustave-Roussy Institute Experience.” International Journal of Radiation Oncology Biology Physics 52 (5): 1257–63. [ PubMed : 11955737 ]
- Mathew B, Sankaranarayanan R, Sunilkumar K B, Kuruvila B, Pisani P. and others. 1997. “Reproducibility and Validity of Oral Visual Inspection by Trained Health Workers in the Detection of Oral Precancer and Cancer.” British Journal of Cancer 76 (3): 390–94. [ PMC free article : PMC2224046 ] [ PubMed : 9252209 ]
- Mathew B, Sankaranarayanan R, Wesley R, Nair M K. 1995. “Evaluation of Mouth Self-Examination in the Control of Oral Cancer.” British Journal of Cancer 71 (2): 397–99. [ PMC free article : PMC2033573 ] [ PubMed : 7841060 ]
- Mehta F S, Gupta P C, Bhonsle R B, Murti P R, Daftary D K, Pindborg J J. 1986. “Detection of Oral Cancer Using Basic Health Workers in an Area of High Oral Cancer Incidence in India.” Cancer Detection and Prevention 9 (3–4):219–25. [ PubMed : 3742502 ]
- Napier S S, Speight P M. 2008. “Natural History of Potentially Malignant Oral Lesions and Conditions: An Overview of the Literature.” Journal of Oral Pathology & Medicine 37 (1): 1–10. [ PubMed : 18154571 ]
- Patel S G, Shah J P. 2005. “TNM Staging of Cancers of the Head and Neck: Striving for Uniformity among Diversity.” CA: A Cancer Journal for Clinicians 55 (4): 242–58. [ PubMed : 16020425 ]
- Patton L L, Epstein J B, Kerr A R. 2008. “Adjunctive Techniques for Oral Cancer Examination and Lesion Diagnosis: A Systematic Review of the Literature.” Journal of the American Dental Association 139 (7): 896–905. [ PubMed : 18594075 ]
- Pavia M, Pileggi C, Nobile C G, Angelillo I F. 2006. “Association between Fruit and Vegetable Consumption and Oral Cancer: A Meta-Analysis of Observational Studies.” American Journal of Clinical Nutrition 83 (5): 1126–34. [ PubMed : 16685056 ]
- Piemonte E D, Lazos J P, Brunotto M. 2010. “Relationship between Chronic Trauma of the Oral Mucosa, Oral Potentially Malignant Disorders and Oral Cancer.” Journal of Oral Pathology & Medicine 39 (7): 513–17. [ PubMed : 20456614 ]
- Prabhu S, Wilson D. 2013. “Human Papillomavirus and Oral Disease: Emerging Evidence: A Review.” Australian Dental Journal 58 (1): 2–10. [ PubMed : 23441786 ]
- Pulte D, Brenner H. 2010. “Changes in Survival in Head and Neck Cancers in the Late 20th and Early 21st Century: A Period Analysis.” Oncologist 15 (9): 994–1001. [ PMC free article : PMC3228039 ] [ PubMed : 20798198 ]
- Radoi L, Paget-Bailly S, Cyr D, Papadopoulos A, Guida F. and others. 2013. “Tobacco Smoking, Alcohol Drinking and Risk of Oral Cavity Cancer by Subsite: Results of a French Population-Based Case-Control Study, the ICARE Study.” European Journal of Cancer Prevention 22 (3): 268–76. [ PubMed : 22976386 ]
- Ramadas K, Lucas E, Thomas G, Mathew B, Balan A. and others. 2008. A Digital Manual for the Early Diagnosis of Oral Neoplasia. Lyon, France: International Agency for Research on Cancer. http://screening .iarc.fr/atlasoral.php .
- Rethman M P, Carpenter W, Cohen E E, Epstein J, Evans C A., and others. 2010. “Evidence-Based Clinical Recommendations Regarding Screening for Oral Squamous Cell Carcinomas.” Journal of the American Dental Association 141 (5): 509–20. [ PubMed : 20436098 ]
- Richards D. 2010. “Does Toluidine Blue Detect More Oral Cancer?” Evidence-Based Dental Practice 11 (4): 104–05. [ PubMed : 21170008 ]
- Ries L A G, Melbert D, Krapcho M, Stinchcomb D G, Howlader N. and others. 2008. Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute. http://seer .cancer.gov/csr/1975-2005 .
- Saba N F, Goodman M, Ward K, Flowers C, Ramalingam S. and others. 2011. “Gender and Ethnic Disparities in Incidence and Survival of Squamous Cell Carcinoma of the Oral Tongue, Base of Tongue, and Tonsils: A Surveillance, Epidemiology and End Results Program-Based Analysis.” Oncology 81 (1): 12–20. [ PMC free article : PMC3186716 ] [ PubMed : 21912193 ]
- Sankaranarayanan R, Ramadas K, Thara S, Muwonge R, Thomas G. and others. 2013. “Long Term Effect of Visual Screening on Oral Cancer Incidence and Mortality in a Randomized Trial in Kerala, India.” Oral Oncology 49 (4): 314–21. [ PubMed : 23265945 ]
- Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S. and others. 2005. “Effect of Screening on Oral Cancer Mortality in Kerala, India: A Cluster-Randomised Controlled Trial.” The Lancet 365 (9475): 1927–33. [ PubMed : 15936419 ]
- Sankaranarayanan R, Swaminathan R. 2011. Cancer Survival in Africa, Asia, the Caribbean and Central America. Scientific Publications No. 162. Lyon, France: International Agency for Research on Cancer. [ PubMed : 21675403 ]
- Sankaranarayanan R, Swaminathan R, Brenner H, Chen K, Chia K S., and others. 2010. “Cancer Survival in Africa, Asia, and Central America: A Population-Based Study.” The Lancet Oncology 11 (2): 165–73. [ PubMed : 20005175 ]
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F. and others. 2009. “EUROCARE-4. Survival of Cancer Patients Diagnosed in 1995–1999.Results and Commentary.” European Journal of Cancer 45 (6): 931–91. [ PubMed : 19171476 ]
- Satyanarayana L, Asthana S. 2008. “Life Time Risk for Development of Ten Major Cancers in India and Its Trends over the Years 1982 to 2000.” Indian Journal of Medical Sciences 62 (2): 35–44. [ PubMed : 18319530 ]
- Scott S E, Rizvi K, Grunfeld E A, Mcgurk M. 2010. “Pilot Study to Estimate the Accuracy of Mouth Self-Examination in an At-Risk Group.” Head & Neck 32 (10): 1393–401. [ PubMed : 20146330 ]
- Sinha D N, Palipudi K M, Rolle I, Asma S, Rinchen S. 2011. “Tobacco Use among Youth and Adults in Member Countries of South-East Asia Region: Review of Findings from Surveys under the Global Tobacco Surveillance System.” Indian Journal of Public Health 55 (3): 169–76. [ PubMed : 22089684 ]
- Sobin L H, Wittekind C. 2002. UICC TNM Classification of Malignant Tumours. Geneva: Union for International Cancer Control.
- Somatunga L C, Sinha D N, Sumanasekera P, Galapatti K, Rinchen S. and others. 2012. “Smokeless Tobacco Use in Sri Lanka.” Indian Journal of Cancer 49 (4): 357–63. [ PubMed : 23442399 ]
- Speight P M, Palmer S, Moles D R, Downer M C, Smith D H. and others. 2006. “The Cost-Effectiveness of Screening for Oral Cancer in Primary Care.” Health Technology Assessment 10 (14): 1–144, Iii-Iv. [ PubMed : 16707071 ]
- Su W W, Yen A M, Chiu S Y, Chen T H. 2010. “A Community-Based RCT for Oral Cancer Screening with Toluidine Blue.” Journal of Dental Research 89 (9): 933–37. [ PubMed : 20525960 ]
- Subramanian S, Sankaranarayanan R, Bapat B, Somanathan T, Thomas G. and others. 2009. “Cost-Effectiveness of Oral Cancer Screening: Results from a Cluster Randomized Controlled Trial in India.” Bulletin of the World Health Organization 87 (3): 200–06. [ PMC free article : PMC2654641 ] [ PubMed : 19377716 ]
- Tseng C H. 2013. “Oral Cancer in Taiwan: Is Diabetes a Risk Factor?” Clinical Oral Investigations 17 (5): 1357–64. [ PubMed : 22895832 ]
- Meij Van Der E H, Bezemer P D, Waal I Van Der. 2002. “Cost-Effectiveness of Screening for the Possible Development of Cancer in Patients with Oral Lichen Planus.” Community Dentistry and Oral Epidemiology 30 (5): 342–51. [ PubMed : 12236825 ]
- Vijayakumar M, Burrah R, Sabitha K S, Nadimul H, Rajani B C. 2011. “To Operate or Not to Operate: N0 Neck in Early Cancer of the Tongue? A Prospective Study.” Indian Journal of Surgical Oncology 2 (3): 172–75. [ PMC free article : PMC3272179 ] [ PubMed : 22942606 ]
- Walsh T, Liu J L, Brocklehurst P, Glenny A M, Lingen M. and others. 2013. “Clinical Assessment to Screen for Oral Cavity Cancer and Potentially Malignant Disorders in Apparently Healthy Adults.” Cochrane Database of Systematic Reviews 11: CD010173. [ PMC free article : PMC7087434 ] [ PubMed : 24258195 ]
- Warnakulasuriya K A, Ekanayake A N, Sivayoham S, Stjernsward J, Pindborg J J., and others. 1984. “Utilization of Primary Health Care Workers for Early Detection of Oral Cancer and Precancer Cases in Sri Lanka.” Bulletin of the World Health Organization 62 (2): 243–50. [ PMC free article : PMC2536304 ] [ PubMed : 6610492 ]
- Warnakulasuriya S, Johnson N W, Waal I Van Der. 2007. “Nomenclature and Classification of Potentially Malignant Disorders of the Oral Mucosa.” Journal of Oral Pathology & Medicine 36 (10): 575–80. [ PubMed : 17944749 ]
- Warnakulasuriya K A, Nanayakkara B G. 1991. “Reproducibility of an Oral Cancer and Precancer Detection Program Using a Primary Health Care Model in Sri Lanka.” Cancer Detection and Prevention 15 (5): 331–34. [ PubMed : 1751941 ]
- Wendt C D, Peters L J, Delclos L, Ang K K, Morrison W H., and others. 1990. “Primary Radiotherapy in the Treatment of Stage I and II Oral Tongue Cancers: Importance of the Proportion of Therapy Delivered with Interstitial Therapy.” International Journal of Radiation Oncology Biology Physics 18 (6): 1287–92. [ PubMed : 2370178 ]
- Woolgar J A. 2006. “Histopathological Prognosticators in Oral and Oropharyngeal Squamous Cell Carcinoma.” Oral Oncology 42 (3): 229–39. [ PubMed : 16150633 ]
- World Cancer Research Fund and AICR (American Institute for Cancer Research). 2007. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR.
- Wrangle J M, Khuri F R. 2007. “Chemoprevention of Squamous Cell Carcinoma of the Head and Neck.” Current Opinion in Oncology 19 (3): 180–87. [ PubMed : 17414634 ]
- Yako-Suketomo H, Matsuda T. 2010. “Comparison of Time Trends in Lip, Oral Cavity and Pharynx Cancer Mortality (1990–2006) between Countries Based on the WHO Mortality Database.” Japanese Journal of Clinical Oncology 40 (11): 1118–19. [ PubMed : 20978186 ]
- Zwetyenga N, Majoufre-Lefebvre C, Siberchicot F, Demeaux H, Pinsolle J. 2003. “[Squamous-cell carcinoma of the tongue: treatment results and prognosis].” Revue de stomatologie et de chirurgie maxillo-faciale 104 (1): 10–17. [ PubMed : 12644785 ]
This work is available under the Creative Commons Attribution 3.0 IGO license (CC BY 3.0 IGO) http://creativecommons.org/licenses/by/3.0/igo . Under the Creative Commons Attribution license, you are free to copy, distribute, transmit, and adapt this work, including for commercial purposes, under the following conditions:
Attribution—Please cite the work as follows: Gelband, H., P. Jha, R. Sankaranarayanan, and S. Horton, editors. 2015. Cancer. Disease Control Priorities , third edition, volume 3. Washington, DC: World Bank. doi:10.1596/978-1-4648-0349-9. License: Creative Commons Attribution CC BY 3.0 IGO
Translations—If you create a translation of this work, please add the following disclaimer along with the attribution: This translation was not created by The World Bank and should not be considered an official World Bank translation. The World Bank shall not be liable for any content or error in this translation.
Adaptations—If you create an adaptation of this work, please add the following disclaimer along with the attribution: This is an adaptation of an original work by The World Bank. Views and opinions expressed in the adaptation are the sole responsibility of the author or authors of the adaptation and are not endorsed by The World Bank.
Third-party content—The World Bank does not necessarily own each component of the content contained within the work. The World Bank therefore does not warrant that the use of any third-party-owned individual component or part contained in the work will not infringe on the rights of those third parties. The risk of claims resulting from such infringement rests solely with you. If you wish to re-use a component of the work, it is your responsibility to determine whether permission is needed for that re-use and to obtain permission from the copyright owner. Examples of components can include, but are not limited to, tables, figures, or images.
All queries on rights and licenses should be addressed to the Publishing and Knowledge Division, The World Bank, 1818 H Street NW, Washington, DC 20433, USA; fax: 202-522-2625; e-mail: gro.knabdlrow@sthgirbup .
- Cite this Page Sankaranarayanan R, Ramadas K, Amarasinghe H, et al. Oral Cancer: Prevention, Early Detection, and Treatment. In: Gelband H, Jha P, Sankaranarayanan R, et al., editors. Cancer: Disease Control Priorities, Third Edition (Volume 3). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2015 Nov 1. Chapter 5. doi: 10.1596/978-1-4648-0349-9_ch5
- PDF version of this title (6.3M)
In this Page
Related information.
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review The Changing Global Burden of Cancer: Transitions in Human Development and Implications for Cancer Prevention and Control. [Cancer: Disease Control Priori...] Review The Changing Global Burden of Cancer: Transitions in Human Development and Implications for Cancer Prevention and Control. Bray F, Soerjomataram I. Cancer: Disease Control Priorities, Third Edition (Volume 3). 2015 Nov 1
- Incidence and survival trends of lip, intra-oral cavity and tongue base cancers in south-east England. [Ann R Coll Surg Engl. 2015] Incidence and survival trends of lip, intra-oral cavity and tongue base cancers in south-east England. Olaleye O, Ekrikpo U, Lyne O, Wiseberg J. Ann R Coll Surg Engl. 2015 Apr; 97(3):229-34.
- Review Costs, Effectiveness, and Cost-Effectiveness of Selected Surgical Procedures and Platforms. [Essential Surgery: Disease Con...] Review Costs, Effectiveness, and Cost-Effectiveness of Selected Surgical Procedures and Platforms. Prinja S, Nandi A, Horton S, Levin C, Laxminarayan R. Essential Surgery: Disease Control Priorities, Third Edition (Volume 1). 2015 Apr 2
- Review Oral cancer prevention and control--the approach of the World Health Organization. [Oral Oncol. 2009] Review Oral cancer prevention and control--the approach of the World Health Organization. Petersen PE. Oral Oncol. 2009 Apr-May; 45(4-5):454-60. Epub 2008 Sep 18.
- Review Cancer in Low- and Middle-Income Countries: An Economic Overview. [Cancer: Disease Control Priori...] Review Cancer in Low- and Middle-Income Countries: An Economic Overview. Horton S, Gauvreau CL. Cancer: Disease Control Priorities, Third Edition (Volume 3). 2015 Nov 1
Recent Activity
- Oral Cancer: Prevention, Early Detection, and Treatment - Cancer Oral Cancer: Prevention, Early Detection, and Treatment - Cancer
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Mouth cancer: presentation, detection and referral in primary dental care
Affiliation.
- 1 School of Dentistry, Cardiff University, Heath Park, Cardiff, CF14 4XY.
- PMID: 30412564
- DOI: 10.1038/sj.bdj.2018.931
Mouth cancer can present as a variety of abnormalities and visible changes affecting the oral mucosa, including ulceration, swelling and areas of erythema. The five-year survival from mouth cancer is poor at approximately 50%. Detection of the cancer while less than 2 cm in diameter with no metastasis greatly improves the outcome for the patient. Although many cancers in the mouth develop from what was previously an apparently normal mucosa, some arise in pre-existing conditions that are therefore regarded as potentially malignant. Regular assessment of the soft tissues within the mouth and the neck for the presence of abnormalities is an essential component of primary dental care. Any persistent and unexplained abnormality requires referral for definitive diagnosis and specialist management.
- Dental Care
- Head and Neck Neoplasms*
- Mouth Mucosa
- Mouth Neoplasms*
- Primary Health Care
- Referral and Consultation

IMAGES
VIDEO
COMMENTS
Oral cancer (OC) is a global health burden with a 5-year survival rate of 50%. It is traditionally defined as oral squamous cell carcinoma due to more than 90% of oral cancers histologically originating in the squamous cells. Early detection of OC improves morbidity accompanying its treatment therefore it is vital for clinicians to recognise the various clinical presentations of OC to ...
The clinical presentation of mouth cancer is highly variable. Regular and thorough examination of the soft tissues to detect any abnormality is an essential aspect of dental primary care.
Oral cancer includes cancers of the oral mucosa, lips, tongue, hard and soft palate, pharynx, and floor of the mouth. The most common types are basal cell carcinoma and squamous cell carcinoma, which can be caused by tobacco use, alcohol, chronic irritation, and human papilloma virus. Staging looks at tumor size and spread to lymph nodes and ...
Oral cancer is one of the most common cancers worldwide and constitutes the third most common cancer in developing countries. It affects lips and other intraoral sites. ... surgery and/or radiotherapy has been used as a curative modality • The aim of treatment depends on the stage of presentation - Early lesions: can completely be treated ...
Oral cancer accounts for roughly two percent of all cancers diagnosed annually in the United States. Approximately 36,500 people will be diagnosed with oral cancer each year and about 7,900 will die from the disease. On average, 61 percent of those with t\ he disease will survive more than 5 years.
mouth-cancer for additional resources Mouth cancers vary significantly in their clinical presentation Screening and early detection2 Screening strategies for asymptomatic individuals Population screening Integrated medical opportunistic screening Screening of high-risk individuals Industrial/ workplace screening Mouth self-examination
Oral cancer is the 11th most common cancer in the world, accounting for an estimated 300,000 new cases and 145,000 deaths in 2012 and 702,000 prevalent cases over a period of five years (old and new cases) (tables 5.1 and 5.2) (Bray and others 2013; Ferlay and others 2013). For this chapter, oral cancers include cancers of the mucosal lip, tongue, gum, floor of the mouth, palate, and mouth ...
Oral cancer - Download as a PDF or view online for free. ... • The stage of disease at presentation is the most important factor. Stage I and II disease has better prognosis( 5 yr. survival 31-100%) whereas advanced stages III, IV have poor prognosis( 5 yr. survival 7-41%)
Included in each Oral Cancer module are handouts with supplemental material to augment the PowerPoint slide presentations. Each module is composed of theory and case-based exercises. The cases have slides with questions related to the salient learning objectives for each case.
Mouth cancer can present as a variety of abnormalities and visible changes affecting the oral mucosa, including ulceration, swelling and areas of erythema. The five-year survival from mouth cancer is poor at approximately 50%. Detection of the cancer while less than 2 cm in diameter with no metastasis greatly improves the outcome for the patient.